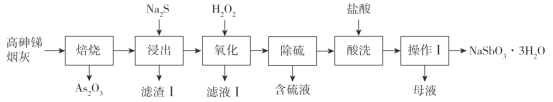
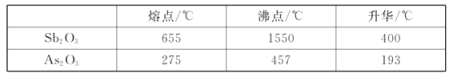
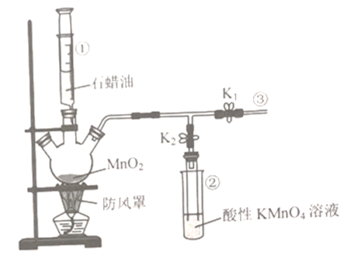
��Ŀ����
����Ŀ���������и��⣺
(1)�ڱ�״���£�CO��CO2�Ļ�����干17.92L������Ϊ27.2g����������������ʵ���֮��Ϊ________ mol������CO2Ϊ________ mol��COռ�������________����������Ħ������Ϊ________��
(2)��100mL��������Һ�У���������ʵ���Ũ��Ϊ0.4mol/L����������ʵ���Ũ��Ϊ0.2mol/L�������м���6.4gͭ�ۣ��ȣ�ʹ���ַ�Ӧ������NO�ڱ�״���µ����Ϊ
_______________
���𰸡�0.8 0.3 62.5% 34g/mol 0.448L
��������
��1�������������ʵ���n=![]() =
=![]() =0.8mol��������̼���Կ���������һ����ԭ�ӵ�һ����̼������˻������Կ���0.8molһ����̼��һЩ��ԭ�ӣ�����m=nM=0.8mol��28g/mol=22.4g�����Щһ����̼������������ԭ�ӵ�����Ϊ27.2g-22.4g=4.8g����ԭ�ӵ����ʵ���Ϊ4.8g��16g/mol=0.3mol���������̼�����ʵ���ҲΪ0.3mol��һ����̼�����ʵ���Ϊ0.8mol-0.3mol=0.5mol�����
=0.8mol��������̼���Կ���������һ����ԭ�ӵ�һ����̼������˻������Կ���0.8molһ����̼��һЩ��ԭ�ӣ�����m=nM=0.8mol��28g/mol=22.4g�����Щһ����̼������������ԭ�ӵ�����Ϊ27.2g-22.4g=4.8g����ԭ�ӵ����ʵ���Ϊ4.8g��16g/mol=0.3mol���������̼�����ʵ���ҲΪ0.3mol��һ����̼�����ʵ���Ϊ0.8mol-0.3mol=0.5mol�����![]() ռ�������
ռ�������![]() ��100%=62.5%����������Ħ������Ϊ
��100%=62.5%����������Ħ������Ϊ![]() =
=![]() =34g/mol��
=34g/mol��
��2��0.4mol/L����������ϡ���ᣬ��˻�����ͭ��Ӧ�����ӷ���ʽΪ![]() ��ͭ���ʵ���Ϊ6.4g��64g/mol=0.1mol���������H+һ���У�0.4mol/L+0.2mol/L��2����0.1L=0.08mol��
��ͭ���ʵ���Ϊ6.4g��64g/mol=0.1mol���������H+һ���У�0.4mol/L+0.2mol/L��2����0.1L=0.08mol��![]() һ����0.4mol/L��0.1L=0.04mol�����Ͽ��Է���H+�������ģ���������ɵ�
һ����0.4mol/L��0.1L=0.04mol�����Ͽ��Է���H+�������ģ���������ɵ�![]() ����Ҫ����
����Ҫ����![]() ���������㣬���ݻ�ѧ������֮�ȣ�0.08mol��
���������㣬���ݻ�ѧ������֮�ȣ�0.08mol��![]() һ��������0.02mol��
һ��������0.02mol��![]() ����Щ
����Щ![]() �ڱ���µ����Ϊ
�ڱ���µ����Ϊ![]() ��
��

 ��У����ϵ�д�
��У����ϵ�д�