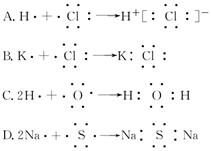��Ŀ����
��14�֣�ij������Ԫ�ص�ԭ������������Ϊ������2�����䵥�ʼɷ������·�Ӧ��
�� + �� �� + �� + ˮ��
�� + �� + ˮ��
��1������ΪNO2��
�ټ����ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
�ڻ������NO2�Ķ�����N2O4�����������£�N2H4����ȼ�ϣ���֪��
N2(g)��2O2(g)��2NO2(g) ��H ����67.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H ����534.0kJ��mol-1
2NO2(g) N2O4(g) ��H ����52.7kJ��mol-1
N2O4(g) ��H ����52.7kJ��mol-1
��д����̬�£�N2H4������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��
___________________________________________________��
�����ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�ء���ȼ�ϵ��ԭ������ͼ��ʾ���ұߵ缫Ϊ �������������������������ߵ缫�Ϸ����ĵ缫��ӦʽΪ ��

��2������ΪSO2��
�ٰ��ҵ�������ͭ�����У��۲쵽�������� ��
��SO2�����ж��������SO2���峣��������NaOH��Һ���գ�д������Һ������Ũ���ɴ�С��˳�� ��
����������ԭ��Ӧ�Ĺ����У�������Ӧ�ͻ�ԭ��Ӧͬʱ�������йط�Ӧ��
SO2��2e����2H2O = SO42����4H����Ӧ��˵��������� ��
�� + ��
 �� + �� + ˮ��
�� + �� + ˮ����1������ΪNO2��
�ټ����ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
�ڻ������NO2�Ķ�����N2O4�����������£�N2H4����ȼ�ϣ���֪��
N2(g)��2O2(g)��2NO2(g) ��H ����67.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H ����534.0kJ��mol-1
2NO2(g)
 N2O4(g) ��H ����52.7kJ��mol-1
N2O4(g) ��H ����52.7kJ��mol-1��д����̬�£�N2H4������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��
___________________________________________________��
�����ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�ء���ȼ�ϵ��ԭ������ͼ��ʾ���ұߵ缫Ϊ �������������������������ߵ缫�Ϸ����ĵ缫��ӦʽΪ ��

��2������ΪSO2��
�ٰ��ҵ�������ͭ�����У��۲쵽�������� ��
��SO2�����ж��������SO2���峣��������NaOH��Һ���գ�д������Һ������Ũ���ɴ�С��˳�� ��
����������ԭ��Ӧ�Ĺ����У�������Ӧ�ͻ�ԭ��Ӧͬʱ�������йط�Ӧ��
SO2��2e����2H2O = SO42����4H����Ӧ��˵��������� ��
| A���÷�ӦΪ������Ӧ |
| B��������Ӧ����ת�Ƶ������ʵ���Ϊ0.05mol����������Һ��PHֵΪ1 |
| C��Fe2(SO4)3��Ʒ��������Һ����ʹ������Ӧ���� |
| D��ͨ��Cl2�ή��SO2��Ư������ |
��1���� C + 4HNO3��Ũ��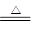 CO2��+ 4NO2��+ 2H2O ��2�֣�
CO2��+ 4NO2��+ 2H2O ��2�֣�
��2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g) ��H����947.6 kJ�� mol��1 ��2�֣�
������ ��2�֣� N2H4��4e����4OH����N2��4H2O ��2�֣�
��2������ɫ������ɫ��ĩ ��2�֣�
�� c(Na+) > c( SO32-) > c(OH-) > c( HSO3-) > c(H+) ��2�֣�
�� B C ��2�֣�
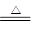 CO2��+ 4NO2��+ 2H2O ��2�֣�
CO2��+ 4NO2��+ 2H2O ��2�֣���2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g) ��H����947.6 kJ�� mol��1 ��2�֣�
������ ��2�֣� N2H4��4e����4OH����N2��4H2O ��2�֣�
��2������ɫ������ɫ��ĩ ��2�֣�
�� c(Na+) > c( SO32-) > c(OH-) > c( HSO3-) > c(H+) ��2�֣�
�� B C ��2�֣�
����������Ϊ������2��������̼Ԫ�ء�
��1���ٱ���NO2��������Ũ���ᣬ��̼��Ӧ������ԭ��Ӧ����ӦʽΪC + 4HNO3��Ũ��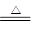 CO2��+ 4NO2��+ 2H2O��
CO2��+ 4NO2��+ 2H2O��
�ڿ����˹���ɵ�Ӧ�á����ݢ�N2(g) + 2O2(g) ��2NO2(g)����N2H4(g) + O2(g) ��N2(g) + 2H2O(g)�͢�NO2(g)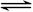 1/2N2O4(g)��֪������ڡ�2���٣��ۼ��õ�2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)�����Է�Ӧ��Ϊ��534.0 kJ/mol��2��67.7 kJ/mol+52.7 kJ/mol����947.6kJ/mol��
1/2N2O4(g)��֪������ڡ�2���٣��ۼ��õ�2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)�����Է�Ӧ��Ϊ��534.0 kJ/mol��2��67.7 kJ/mol+52.7 kJ/mol����947.6kJ/mol��
���Ҳ�ͨ����������������������Ǹ�������ʧȥ���ӣ�����������Ӧ���缫��ӦʽΪN2H4��4e����4OH����N2��4H2O��
��2������ΪSO2��������Ũ���ᣬ����CO2����Ũ���������ˮ�ԣ��������յ����е�ˮ���ӣ�������ˮ����ͭ��������ɫ������ɫ��ĩ��
��SO2���������Ʒ�Ӧ�����������ƣ���������ˮ�⣬��Һ�Լ��ԡ�����Ũ�ȴ�С˳��Ϊc(Na+) > c( SO32-) > c(OH-) > c( HSO3-) > c(H+)��
�۷�Ӧʧȥ���ӣ�������������Ӧ��ÿת��2mol���ӣ�������4mol�����ӣ�������ת�Ƶ������ʵ���Ϊ0.05mol�������������ӵ����ʵ���Ϊ0.1mol������Һ�������δ֪�ģ�����������pH��B����ȷ��SO2ʹƷ����Һ��ɫ������������ԭ��Ӧ��C����ȷ���������������ԣ�SO2���л�ԭ�ԣ����߷���������ԭ��Ӧ��������������ᣬ����Ư���ԣ�D��ȷ��
��1���ٱ���NO2��������Ũ���ᣬ��̼��Ӧ������ԭ��Ӧ����ӦʽΪC + 4HNO3��Ũ��
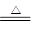 CO2��+ 4NO2��+ 2H2O��
CO2��+ 4NO2��+ 2H2O���ڿ����˹���ɵ�Ӧ�á����ݢ�N2(g) + 2O2(g) ��2NO2(g)����N2H4(g) + O2(g) ��N2(g) + 2H2O(g)�͢�NO2(g)
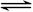 1/2N2O4(g)��֪������ڡ�2���٣��ۼ��õ�2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)�����Է�Ӧ��Ϊ��534.0 kJ/mol��2��67.7 kJ/mol+52.7 kJ/mol����947.6kJ/mol��
1/2N2O4(g)��֪������ڡ�2���٣��ۼ��õ�2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)�����Է�Ӧ��Ϊ��534.0 kJ/mol��2��67.7 kJ/mol+52.7 kJ/mol����947.6kJ/mol�����Ҳ�ͨ����������������������Ǹ�������ʧȥ���ӣ�����������Ӧ���缫��ӦʽΪN2H4��4e����4OH����N2��4H2O��
��2������ΪSO2��������Ũ���ᣬ����CO2����Ũ���������ˮ�ԣ��������յ����е�ˮ���ӣ�������ˮ����ͭ��������ɫ������ɫ��ĩ��
��SO2���������Ʒ�Ӧ�����������ƣ���������ˮ�⣬��Һ�Լ��ԡ�����Ũ�ȴ�С˳��Ϊc(Na+) > c( SO32-) > c(OH-) > c( HSO3-) > c(H+)��
�۷�Ӧʧȥ���ӣ�������������Ӧ��ÿת��2mol���ӣ�������4mol�����ӣ�������ת�Ƶ������ʵ���Ϊ0.05mol�������������ӵ����ʵ���Ϊ0.1mol������Һ�������δ֪�ģ�����������pH��B����ȷ��SO2ʹƷ����Һ��ɫ������������ԭ��Ӧ��C����ȷ���������������ԣ�SO2���л�ԭ�ԣ����߷���������ԭ��Ӧ��������������ᣬ����Ư���ԣ�D��ȷ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ