��Ŀ����
1������һ�ֺܻ��õ�Ԫ�أ������˵����������γ�-2��+6��+4��+2��+1�۵Ļ����I�����������ƣ�Na2S2O5���dz��õ�ʳƷ��������֮һ������ǿ�ҵ�SO2��ζ��ˮ��Һ����NaHSO3�����ԣ����ÿ��������������ʸò�Ʒ���ܾô森ij�о�С�������ͼװ�ã�ʵ��ǰ�ѳ���װ���ڵĿ�������ȡNa2S2O5��
 ��1��װ�� I�е�Ũ����ܣ��ܻ��ܣ���ϡ������棬ԭ���Ƕ�������������ˮ���ʲ�����ϡ���ᣮ
��1��װ�� I�е�Ũ����ܣ��ܻ��ܣ���ϡ������棬ԭ���Ƕ�������������ˮ���ʲ�����ϡ���ᣮ��2��װ�â�����Na2S2O5����������Ҫ����������ľ��壬�ɲ�ȡ�ķ��뷽���ǹ��ˣ�
��3��װ�â����ڴ���β�������������ڻ���β������װ�ú�ҩƷ��
��4������Na2S2O5�����ڿ����б��ʵ�ʵ�鷽����ȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ����������ᣬ���ٵ����Ȼ�����Һ���а�ɫ�������ɣ���˵�����ʣ�
����һ����Ļ�����Na2S2O3����Һ�������ڲⶨ��Һ��ClO2�ĺ������ɽ�������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00mL��ϡ�ͳ�100mL������
����2����ȡV1mL�������뵽��ƿ�У�����������pH��2.0������������KI���壬ҡ�ȣ��ڰ�������30���ӣ�����֪��ClO2+I-+H+-I2+Cl-+H2O δ��ƽ��
����3���Ե�����Һ��ָʾ������c mol•L-1Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪��I2+2S2O32-=2I-+S4O62-��
��1��ȷ��ȡ10.00mL ClO2��Һ�IJ�����������ʽ�ζ��ܣ�
��2��ȷ���ζ��յ������Ϊ�μ����һ��Na2S2O3��Һʱ����Һ�պ�����ɫ��Ϊ��ɫ���ұ���30s���䣮
��3������������������ԭClO2��Һ�����ʵ���Ũ��Ϊ$\frac{2c{V}_{2}}{{V}_{1}}$mol•L-1���ú���ĸ�Ĵ���ʽ��ʾ��
��4�����в����ᵼ�²ⶨ���ƫ�ߵ���AC��
A��δ�ñ�Ũ�ȵ�Na2S2O3��Һ��ϴ�ζ���
B���ζ�ǰ��ƿ������ˮ
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ
D���ζ�Ӧ�����Ի������Ի����н��У�����Һ�ʼ���
E���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ�
���� I����1��װ�� I�еķ�Ӧ������������������������ˮ���ݴ��жϣ�
��2��װ�â���ΪNa2S2O5�������Һ�����ݷ�������Һ��ͨ���õķ������⣻
��3��װ�â����ڴ���β��������δ��Ӧ�Ķ�������Ӧ��ֹ�������Ҳ��ܴ�����ȫ�ܱջ����У�
��4��Na2S2O5�����ڿ����б������������ƣ�ͨ��������������ӿ��ж��Ƿ���ʣ�
��1��ClO2��Һ����ǿ�����ԣ�
��2���ζ��յ�ʱNa2S2O3��Һ����ȫ����ԭ���Ե�����Һ��ָʾ������Һ��ɫ��ȥ��
��3���ɷ���ʽ2ClO2+10I-+8H+=5I2+2Cl-+4H2O��I2+2S2O32-�T2I-+S4O62-�ù�ϵʽClO2��5S2O32-��n��S2O32-��=cV2��10-3mol������V1mL ClO2����Һ�к��е�ClO2�����ʵ���Ϊ2cV2��10-4mol������c=$\frac{n}{V}$�����ԭClO2��Һ�����ʵ���Ũ�ȣ�
��4��A��δ�ñ�Ũ�ȵ�Na2S2O3��Һ��ϴ�ζ��ܣ��ᵼ�±�ҺŨ�ȱ�С����ȥ��Һ�����ƫ��
B���ζ�ǰ��ƿ������ˮ����ʵ����Ӱ�죻
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ���ᵼ�±�Һ�����ƫ��
D���ζ�Ӧ�����Ի������Ի����н��У�����Һ�ʼ��ԣ��ᵼ����ȥ�ı�Һ�����ƫС��
E���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ�������Һ�����������ƫС��
��� �⣺I����1��װ�� I�еķ�Ӧ������������������������ˮ�����Բ�����ϡ�������Ũ���ᣬ
�ʴ�Ϊ�����ܣ���������������ˮ���ʲ�����ϡ���
��2��װ�â���ΪNa2S2O5�������Һ����������Һ��ͨ���õķ����ǹ��ˣ�
�ʴ�Ϊ�����ˣ�
��3��װ�â����ڴ���β��������δ��Ӧ�Ķ�������Ӧ��ֹ�������Ҳ��ܴ�����ȫ�ܱջ����У�����β������װ�ú�ҩƷΪ ��
��
�ʴ�Ϊ�� ��
��
��4��Na2S2O5�����ڿ����б������������ƣ�ͨ��������������ӿ��ж��Ƿ���ʣ�ʵ�鷽����ȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ����������ᣬ���ٵ����Ȼ�����Һ���а�ɫ�������ɣ���˵�����ʣ�
�ʴ�Ϊ��ȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ����������ᣬ���ٵ����Ȼ�����Һ���а�ɫ�������ɣ���˵�����ʣ�
��1��ClO2��Һ����ǿ�����ԣ�����ȷ��ȡ10.00 mL ClO2��Һ�IJ�����������ʽ�ζ��ܣ�
�ʴ�Ϊ����ʽ�ζ��ܣ�
��2���ζ��յ�ʱNa2S2O3��Һ����ȫ����ԭ���Ե�����Һ��ָʾ������Һ��ɫ��ȥ���ζ��յ������Ϊ�μ����һ��Na2S2O3��Һʱ����Һ�պ�����ɫ��Ϊ��ɫ���ұ���30s���䣬
�ʴ�Ϊ���μ����һ��Na2S2O3��Һʱ����Һ�պ�����ɫ��Ϊ��ɫ���ұ���30s���䣻
��3���ɷ���ʽ2ClO2+10I-+8H+=5I2+2Cl-+4H2O��I2+2S2O32-�T2I-+S4O62-�ù�ϵʽClO2��5S2O32-��n��S2O32-��=cV2��10-3mol������V1mL ClO2����Һ�к��е�ClO2�����ʵ���Ϊ2cV2��10-4mol����10mL��ԭ��Һ����ClO2�����ʵ���Ϊ��2cV2��10-4mol��$\frac{100mL}{V{\;}_{1}}$=$\frac{2c{V}_{2}}{{V}_{1}}$��10-2mol������ԭClO2��Һ�����ʵ���Ũ��Ϊ��$\frac{\frac{2c{V}_{2}}{{V}_{1}}��1{0}^{-2}}{0.01L}$=$\frac{2c{V}_{2}}{{V}_{1}}$mol/L��
�ʴ�Ϊ��$\frac{2c{V}_{2}}{{V}_{1}}$��
��4��A��δ�ñ�Ũ�ȵ�Na2S2O3��Һ��ϴ�ζ��ܣ��ᵼ�±�ҺŨ�ȱ�С����ȥ��Һ�����ƫ�����Բⶨ���ƫ�ߣ�
B���ζ�ǰ��ƿ������ˮ����ʵ����Ӱ�죻
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ���ᵼ�±�Һ�����ƫ�����Բⶨ���ƫ�ߣ�
D���ζ�Ӧ�����Ի������Ի����н��У�����Һ�ʼ��ԣ��ᵼ����ȥ�ı�Һ�����ƫС�����Բⶨ���ƫ�ͣ�
E���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ�������Һ�����������ƫС�����Բⶨ���ƫ�ͣ�
�ʴ�Ϊ��AC��
���� ���⿼��������ʵ�鷽������ơ���ѧʵ������������������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷʵ��ԭ������ѧʵ�������������Ϊ���ؼ��������ۺ��Խ�ǿ����ֿ�����ѧ���ķ������������������Ӧ�û���֪ʶ��������

| A�� | ����ռ���ڼ� | |
| B�� | Ư�ۡ�С�մ����ڴ����� | |
| C�� | �Ȼ�李������ᶼ���ڵ���� | |
| D�� | �ϳ���ά���ά�����������ǽ������� |
�仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ�����
| T/�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=$\frac{c��CO����c��{H}_{2}O��}{c��C{O}_{2}����c��{H}_{2}��}$���÷�Ӧ������Ӧ�����H��0���������������
��2���������ݶ�Ӧ�Ļ�ѧƽ��״̬�������¶���830��
��3���������������䣬1000��ʱ�����������c��CO��=0.060mol•L-1������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬��ѡ���ǻ����ʱ����ѧ��Ӧ������v��С��v�棨ѡ����ڡ�С�ڻ���ڣ�����ԭ����$\frac{c��CO����c��{H}_{2}O��}{c��C{O}_{2}����c��{H}_{2}��}$=2.25��1.7��˵����ʱc��CO������ƽ��״̬��Ũ�ȣ�����v���棩��v��������
| Ԫ�� | H | Li | Be | B | C | N | O | F |
| �縺�� | 2.1 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.7 | 2.1 | 2.3 | 3.0 | 0.8 |
��1�����Ƹ�Ԫ�صĵ縺�Ե�ȡֵ��Χ��0.8��X��1.2��
��2��������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�صĵ縺�Բ�ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3���γɵĻ�ѧ��������Ϊ���ۼ�����������AlCl3��Cl��Al�ĵ縺�Բ�ֵΪ1.5����Br�ĵ縺��С��Cl������AlBr3����Ԫ�صĵ縺�Բ�ֵС��1.5��
��3��ij����������к���S-N��������Ϊ�ù��õ��Ӷ�ƫ����Nԭ�ӣ���Ԫ�ط��ţ�
| A�� | ����ͭ��Һ�м�������������ҺCu2++SO42-+Ba2++2OH-�TBaSO4��+Cu��OH��2�� | |
| B�� | ͭƬ������������Һ�У�Cu+Ag+�TCu2++Ag | |
| C�� | ��̼��������Һ�м���������Һ��HCO3-+H+�TCO2��+H2O | |
| D�� | �ð�ˮ���Ȼ�����Ӧ�Ʊ���������������Al3++3OH-�TAl��OH��3�� |
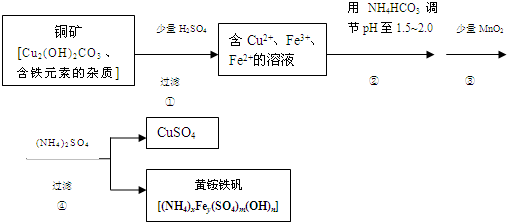
 ��Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
��Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���