��Ŀ����
����Ŀ�������ᣨ��H3R��ʾ����������ϴ���������£���0.1mol��L1 H3R��Һ�м���������NaOH���壨������Һ����ı仯����H3R��H2R��HR2��R3�ĺ�����pH�Ĺ�ϵ��ͼ��ʾ��������ȷ����

A. ͼ��b���߱�ʾHR2�ı仯
B. HR2�ĵ��볣��Ka3��106
C. pH��7ʱ��c(Na+)��c(H2R)+c(HR2)+c(R3)
D. pH��5ʱ��c(H2R)+c(HR2)+c(R3)��0.1mol��L1
���𰸡�B
��������
��������������ʣ��ܷ����������룺H3R![]() H2R-
H2R-![]() H+��H2R-
H+��H2R-![]() HR2-
HR2-![]() H+��HR2-
H+��HR2-![]() R3--
R3--![]() H+����pH=1ʱ��H3R����̶ȶ���С����Һ�е���������ΪH3R��������ҺpH������һ�����ڶ��������������뿪ʼ����������������a��b��c��d���������ֱ�ΪH3R��H2R-��HR2-��R3-�ĺ����仯���ߡ�
H+����pH=1ʱ��H3R����̶ȶ���С����Һ�е���������ΪH3R��������ҺpH������һ�����ڶ��������������뿪ʼ����������������a��b��c��d���������ֱ�ΪH3R��H2R-��HR2-��R3-�ĺ����仯���ߡ�
A.��������������֪����ͼ��b���߱�ʾH2R���ı仯����A����
B.��ͼ���Կ�������pH=6 ʱ, HR2-![]() R3-
R3-![]() H+��ƽ����ϵ�У�c(R3-)= c(HR2��)��c(H+)=1
H+��ƽ����ϵ�У�c(R3-)= c(HR2��)��c(H+)=1![]() mol/L��HR2���ĵ��볣��Ka3= c(H+)
mol/L��HR2���ĵ��볣��Ka3= c(H+)![]() c(R3-)/ c(HR2��)=c(H+)= 1
c(R3-)/ c(HR2��)=c(H+)= 1![]() ����B��ȷ��
����B��ȷ��
C.pH=7 ʱ����Һ�����ԣ�c(H+)=c(OH-)�����ݵ���غ㣬c(Na+)=c(H2R-)+2c(HR2��)+3c(R3��)����C����
D.����ԭ��ҺH3R��Ũ��Ϊ0.1mol��L��1����������NaOH��pH =5����ʱ��Һ������������c(H2R�� )+c(HR2��)+c(R3��)![]() 0. 1mol��L��1����D����
0. 1mol��L��1����D����
�����ΪB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ�������ͼ����Ϣ���ܳ��˵����Ӧ�Ļ�ѧ��Ӧ�Ƿ��ȷ�Ӧ����
A | B | C | D | |
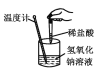
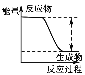
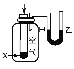
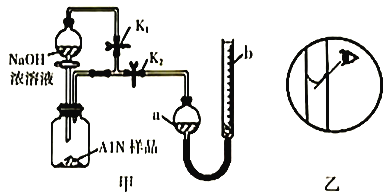
ͼʾ |
|
|
|
|
��� ��Ϣ | �¶ȼƵ�ˮ������������ | ��Ӧ������������������������ | ��Ӧ��ʼ�״�Һ������Ҵ�Һ�� | ��Ӧ��ʼ����Ͳ���������ƶ� |
A.AB.BC.CD.D