��Ŀ����
��ij�о���ѧϰС����10g������ȡCuO����֤��CuO���Դ�H2O2�ķֽⷴӦ��
��1�������Ʊ�CuO��ʵ�鲽�����£������ȱ�ٵIJ��裺
�ٳ�ȡ10g����������С�ձ��У��ټ�ˮ�ܽ⣻����С�ձ��еμ�NaOH��Һ���������������������þƾ�����ʯ�����ϼ���С�ձ�������������ȫ��ɫ���ܽ����ϻ������ˣ�ϴ�ӣ�______��Ȼ����ϸ���ݼ�����ϴ���Ƿ���ȫ�IJ�����______��
��2����ͼ1��ʾ�����������ʵ�鷽����֤��CuO�ܴ�7%H2O2��Һ�ķֽ⣬����MnO2�Ĵ�Ч�����бȽϣ�
�ٴ����ԭ������ʵ���еĴ���������______��
��Ϊ̽��CuO��ʵ��b���Ƿ�������ã�����
a�Ƚ��⣬��Ӧ��������ʵ�飨����д�����������
A��֤��CuO��ѧ�����ڷ�Ӧǰ���Ƿ�ı䣻
B��______��
��Ħ����[��NH4��2SO4-FeSO4-6H2O]
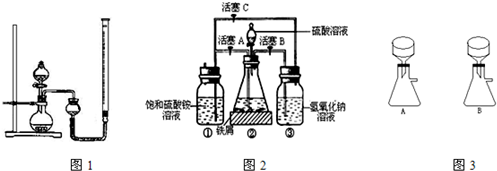
�ڿ����б�һ���������ȶ����ǻ�ѧ�����г��õĻ�ԭ����ij�о���ѧϰС����ͼ2��ʾ��ʵ��װ������ȡĦ���Σ�ʵ�鲽�����£��ش��������⣺
��1����30%��NaOH��Һ�ͷ���м�����������ۡ����⡢FeS�ȣ���ϡ���С���ȴ�����룬���������NaOH��Һװ����У�
��2�����������ڵķ�Ӧ������������ͨ��������Ӧ�رջ���______������______������ĸ��������������ͨ��������Ŀ����______��
��3������ƿ�е���м�췴Ӧ��ʱ���رջ���B��C������A�����������������Ὣ��ƿ�е����������������ٲ���δ��Ӧ��ϡ���ᣩѹ�������������Һ�ĵײ����ڳ����·���һ��ʱ�䣬�Լ�ƿ�ײ����ᾧ����������泥����ˣ��Ƶ���������茶��壮ͼ3�dz���װ�õ�һ���֣�������ȷ����______������A��B��
��4��Ϊ��ȷ����Ʒ���������ӵĺ������о�С���õζ������ⶨ����ȡ��Ʒ24.50g���100mL��Һ��ȡ��10.00mL��0.1000mol-L-1KMnO4������Һ�ζ�������KMnO4��Һ10.00mL��
��֪���������Fe2+�����ӷ���ʽΪ��MnO
+5Fe2++8H+=Mn2++5Fe3++4H2O
�����Ʒ��Ħ���ε���������______����NH4��2SO4-FeSO4-6H2O����Է�������Ϊ392��
��1�������Ʊ�CuO��ʵ�鲽�����£������ȱ�ٵIJ��裺
�ٳ�ȡ10g����������С�ձ��У��ټ�ˮ�ܽ⣻����С�ձ��еμ�NaOH��Һ���������������������þƾ�����ʯ�����ϼ���С�ձ�������������ȫ��ɫ���ܽ����ϻ������ˣ�ϴ�ӣ�______��Ȼ����ϸ���ݼ�����ϴ���Ƿ���ȫ�IJ�����______��
��2����ͼ1��ʾ�����������ʵ�鷽����֤��CuO�ܴ�7%H2O2��Һ�ķֽ⣬����MnO2�Ĵ�Ч�����бȽϣ�
| ʵ����� | ˫��ˮ��� | ���� | �������� |
| a | 15mL | �� | |
| b | 15mL | 0.5g CuO | |
| c | 15mL | 0.5g MnO2 |
��Ϊ̽��CuO��ʵ��b���Ƿ�������ã�����
a�Ƚ��⣬��Ӧ��������ʵ�飨����д�����������
A��֤��CuO��ѧ�����ڷ�Ӧǰ���Ƿ�ı䣻
B��______��
��Ħ����[��NH4��2SO4-FeSO4-6H2O]

�ڿ����б�һ���������ȶ����ǻ�ѧ�����г��õĻ�ԭ����ij�о���ѧϰС����ͼ2��ʾ��ʵ��װ������ȡĦ���Σ�ʵ�鲽�����£��ش��������⣺
��1����30%��NaOH��Һ�ͷ���м�����������ۡ����⡢FeS�ȣ���ϡ���С���ȴ�����룬���������NaOH��Һװ����У�
��2�����������ڵķ�Ӧ������������ͨ��������Ӧ�رջ���______������______������ĸ��������������ͨ��������Ŀ����______��
��3������ƿ�е���м�췴Ӧ��ʱ���رջ���B��C������A�����������������Ὣ��ƿ�е����������������ٲ���δ��Ӧ��ϡ���ᣩѹ�������������Һ�ĵײ����ڳ����·���һ��ʱ�䣬�Լ�ƿ�ײ����ᾧ����������泥����ˣ��Ƶ���������茶��壮ͼ3�dz���װ�õ�һ���֣�������ȷ����______������A��B��
��4��Ϊ��ȷ����Ʒ���������ӵĺ������о�С���õζ������ⶨ����ȡ��Ʒ24.50g���100mL��Һ��ȡ��10.00mL��0.1000mol-L-1KMnO4������Һ�ζ�������KMnO4��Һ10.00mL��
��֪���������Fe2+�����ӷ���ʽΪ��MnO
| -4 |
�����Ʒ��Ħ���ε���������______����NH4��2SO4-FeSO4-6H2O����Է�������Ϊ392��
��1���ܡ��������ˣ�ϴ�ӣ�����������ϸ��
�ʴ�Ϊ�����
�ݡ��ɹ������̿�֪���е�CuO���ܺ�������ͭ�����������ʣ�ȡ���ϴ��Һ������BaCl2��Һ�������ǣ�֤��������ϴ����
�ʴ�Ϊ��ȡ���ϴ��Һ������BaCl2��Һ�������ǣ�֤��������ϴ����
��2�����ɱ�����Ϣ��֪��˫��ˮ������һ�����Ƚϴ���������ͬʱ�������ɵ��������Խ�ࣨ�������ͬ�����������Ҫ��ʱ��Խ�̣���������Խǿ������Ӧ����������Ϊ��ͬʱ���ڲ��������������������ͬ�����������Ҫ��ʱ�䣩��
�ʴ�Ϊ����ͬʱ���ڲ��������������������ͬ�����������Ҫ��ʱ�䣩��
�ڴ����ڷ�Ӧǰ��ѧ���ʲ��䣬�������䣮�ʻ���CuO�������ڷ�Ӧǰ���Ƿ����ı䣮
�ʴ�Ϊ��CuO�������ڷ�Ӧǰ���Ƿ����ı䣮
��2����װ��ͼ��֪������������ͨ��������Ӧ�رջ���A������B��C��
�������Һ�е��ܽ�O2��������Һ���ϲ���O2��Fe2+�ױ�����ΪFe3+��ͨ�����������������Һ�е��ܽ�O2��������Һ���ϲ���O2����ֹFe2+������ΪFe3+��
�ʴ�Ϊ��A��B��C�������������Һ�е��ܽ�O2��������Һ���ϲ���O2����ֹFe2+������ΪFe3+��
��3������©���ľ��¿�б��Ӧ�����ƿ��֧����ԣ������ڼ�ѹ���ˣ���A��ȷ��B����
��ѡ��A��
��4����24.50g��Ʒ��Fe2+�����ʵ���Ϊxmol����
5Fe2+������������MnO4-��
5 1
xmol 0.01L��0.1000mol/L��10
����x=
=0.05mol��
����24.50g��Ʒ�У�NH4��2SO4?FeSO4?6H2O��Ϊ0.05mol��
����24.50g��Ʒ�У�NH4��2SO4?FeSO4?6H2O������Ϊ0.05mol��392g/mol=19.6g��
����24.50g��Ʒ�У�NH4��2SO4?FeSO4?6H2O����������Ϊ
��100%=80%��
�ʴ�Ϊ��80%��
�ʴ�Ϊ�����
�ݡ��ɹ������̿�֪���е�CuO���ܺ�������ͭ�����������ʣ�ȡ���ϴ��Һ������BaCl2��Һ�������ǣ�֤��������ϴ����
�ʴ�Ϊ��ȡ���ϴ��Һ������BaCl2��Һ�������ǣ�֤��������ϴ����
��2�����ɱ�����Ϣ��֪��˫��ˮ������һ�����Ƚϴ���������ͬʱ�������ɵ��������Խ�ࣨ�������ͬ�����������Ҫ��ʱ��Խ�̣���������Խǿ������Ӧ����������Ϊ��ͬʱ���ڲ��������������������ͬ�����������Ҫ��ʱ�䣩��
�ʴ�Ϊ����ͬʱ���ڲ��������������������ͬ�����������Ҫ��ʱ�䣩��
�ڴ����ڷ�Ӧǰ��ѧ���ʲ��䣬�������䣮�ʻ���CuO�������ڷ�Ӧǰ���Ƿ����ı䣮
�ʴ�Ϊ��CuO�������ڷ�Ӧǰ���Ƿ����ı䣮
��2����װ��ͼ��֪������������ͨ��������Ӧ�رջ���A������B��C��
�������Һ�е��ܽ�O2��������Һ���ϲ���O2��Fe2+�ױ�����ΪFe3+��ͨ�����������������Һ�е��ܽ�O2��������Һ���ϲ���O2����ֹFe2+������ΪFe3+��
�ʴ�Ϊ��A��B��C�������������Һ�е��ܽ�O2��������Һ���ϲ���O2����ֹFe2+������ΪFe3+��
��3������©���ľ��¿�б��Ӧ�����ƿ��֧����ԣ������ڼ�ѹ���ˣ���A��ȷ��B����
��ѡ��A��
��4����24.50g��Ʒ��Fe2+�����ʵ���Ϊxmol����
5Fe2+������������MnO4-��
5 1
xmol 0.01L��0.1000mol/L��10
����x=
| 0.01L��0.1000mol/L��10 ��5 |
| 1 |
����24.50g��Ʒ�У�NH4��2SO4?FeSO4?6H2O��Ϊ0.05mol��
����24.50g��Ʒ�У�NH4��2SO4?FeSO4?6H2O������Ϊ0.05mol��392g/mol=19.6g��
����24.50g��Ʒ�У�NH4��2SO4?FeSO4?6H2O����������Ϊ
| 19.6g |
| 24.50g |
�ʴ�Ϊ��80%��

��ϰ��ϵ�д�
�����Ŀ
