��Ŀ����
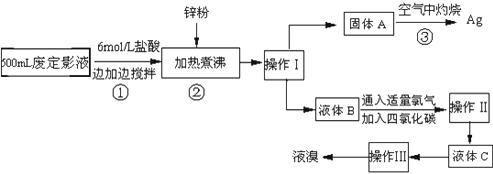
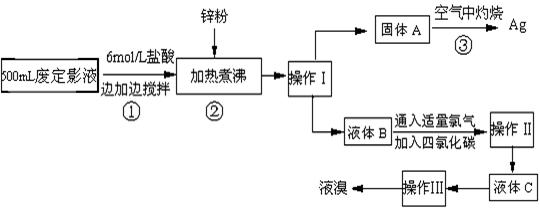
�����õķ϶�ӰҺ�к���Na+��[Ag��S2O3��2]3-��Br-�����ӣ�ij�о���ѧϰС����ͨ������ʵ�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е������壮
��֪��4H++2[Ag��S2O3��2]3-=Ag2S��+3S��+3SO2��+SO42-+2H2O
��1������п�۵�Ŀ���ǽ���Һ�е������廯����ԭ�������÷�Ӧ�����ӷ�Ӧ����ʽΪ
��2���������������
��3��Һ��B�г�����Br-������SO42-��������Һ�д���SO42-�IJ�����
��4��ͨ���������̻������������У�����ɫ��ѧ��Ҫ�����ڵIJ���Ϊ
��������1��п���廯����Ӧ������п���Ӻ������ʣ�2AgBr+Zn=Ag+Zn2++2Br-��
��2�����ݲ����Թ����Һ�����ķ������ǣ��������ܵ�Һ����뷽�����ǣ���������õ����������ǣ�
��3�����ݼ���������ķ�����ɣ�
��4���������ջ�����ж��Ķ���������Ⱦ������
��2�����ݲ����Թ����Һ�����ķ������ǣ��������ܵ�Һ����뷽�����ǣ���������õ����������ǣ�
��3�����ݼ���������ķ�����ɣ�
��4���������ջ�����ж��Ķ���������Ⱦ������
����⣺��1��п�û����ķ�Ӧ����Ӧ�����ӷ���ʽ�ǣ�2AgBr+Zn=Ag+Zn2++2Br-��
�ʴ��ǣ�2AgBr+Zn=Ag+Zn2++2Br-��
��2��������Ҫ�����������õ����˲������������Ƿ���������Ȼ�̼��Һ��ˮ���õ���Һ�������������Ǵ�Һ�������з�����壬�õ��������������������̨���ƾ��ơ���ƿ����Һ����������ƿ���¶ȼƣ������ܵȣ�
�ʴ��ǣ����ˣ���Һ������ȡ��Һ����������ƿ���¶ȼƣ������ܣ�
��3��������������ӵķ����ǣ�ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����֤����SO42-��
�ʴ��ǣ�ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����֤����SO42-��
��4������A�����������ջ�����ж����ʶ���������ɴ�����Ⱦ��
�ʴ��ǣ��ڿ��������ղ�����SO2�ж�����Ⱦ������
�ʴ��ǣ�2AgBr+Zn=Ag+Zn2++2Br-��
��2��������Ҫ�����������õ����˲������������Ƿ���������Ȼ�̼��Һ��ˮ���õ���Һ�������������Ǵ�Һ�������з�����壬�õ��������������������̨���ƾ��ơ���ƿ����Һ����������ƿ���¶ȼƣ������ܵȣ�
�ʴ��ǣ����ˣ���Һ������ȡ��Һ����������ƿ���¶ȼƣ������ܣ�
��3��������������ӵķ����ǣ�ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����֤����SO42-��
�ʴ��ǣ�ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����֤����SO42-��
��4������A�����������ջ�����ж����ʶ���������ɴ�����Ⱦ��
�ʴ��ǣ��ڿ��������ղ�����SO2�ж�����Ⱦ������
���������⿼����������Ļ��ռ��Ͻ𱣻��������ؼ���Ҫ֪�����˷���������ص㣬�˽⻯ѧ����ʽ��д������Һϡ�͵ķ������õ�����������Ϥ�û���Ӧ���ص㣬�����Ѷ��еȣ�

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ

 .
.