��Ŀ����
������2L���ݵ������У��ֱ�����ӦA(g)+3B(g) 2C(g)��5min���������Ӧ��ƽ��״̬,���м�������A�����ʵ���Ϊ0.4mol��
2C(g)��5min���������Ӧ��ƽ��״̬,���м�������A�����ʵ���Ϊ0.4mol��
����˵����ȷ���ǣ� ��
A������5min��C�ķ�Ӧ����Ϊ0.12mol/(L��min)
B���ﵽƽ��ʱ������A��Ũ���Ǽ��е�2��
C�����з�Ӧ��ƽ�ⳣ�����ڼ��з�Ӧ��ƽ�ⳣ��
D���ﵽƽ��ʱ��������������C�����ʵ����ٷֺ������
 2C(g)��5min���������Ӧ��ƽ��״̬,���м�������A�����ʵ���Ϊ0.4mol��
2C(g)��5min���������Ӧ��ƽ��״̬,���м�������A�����ʵ���Ϊ0.4mol��| | ��Ӧǰ�����ʵ����ʵ���/mol | ||
| A | B | C | |
| �� | 1 | 3 | 0 |
| �� | 0 | 0 | 2 |
| �� | 1.5 | 4.5 | 1 |
A������5min��C�ķ�Ӧ����Ϊ0.12mol/(L��min)
B���ﵽƽ��ʱ������A��Ũ���Ǽ��е�2��
C�����з�Ӧ��ƽ�ⳣ�����ڼ��з�Ӧ��ƽ�ⳣ��
D���ﵽƽ��ʱ��������������C�����ʵ����ٷֺ������
D
�������൱����1molA��3molB�����Լ����ǵ�Ч�ģ�D��ȷ��ƽ��ʱ��������A�����ʵ���Ϊ0.4mol�����������У�����C��0.8mol������C�ķ�Ӧ������0.8mol��2L��5min��0.08mol/(L��min)��A����ȷ���������൱��2molA��6molB����������Ӧ�������С�Ŀ��淴Ӧ������ƽ��ʱ����A��Ũ��С�ڼ��е�2����B����ȷ��ƽ�ⳣ�����¶��й�ϵ�����Ա��ͼ���ƽ�ⳣ������ͬ�ģ�C����ȷ����ѡD��

��ϰ��ϵ�д�
�����Ŀ

 N2 + 3H2����ij�¶��µ�ƽ�ⳣ��Ϊ0.25����ô���ڴ������£����ĺϳɷ�Ӧ1/2 N2 + 3/2 H2
N2 + 3H2����ij�¶��µ�ƽ�ⳣ��Ϊ0.25����ô���ڴ������£����ĺϳɷ�Ӧ1/2 N2 + 3/2 H2 I2 + I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4�����Ϻ�ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
I2 + I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4�����Ϻ�ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� CO��g����3H2��g������H����206 kJ/mol��һ�������������Ϊ1 L���ܱ������г���1 mol CH4��1 mol H2O�����CH4��g����CO��g����Ũ����ʱ��仯������ͼ��ʾ������˵����ȷ���� �� ��
CO��g����3H2��g������H����206 kJ/mol��һ�������������Ϊ1 L���ܱ������г���1 mol CH4��1 mol H2O�����CH4��g����CO��g����Ũ����ʱ��仯������ͼ��ʾ������˵����ȷ���� �� ��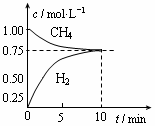
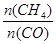 ����
���� CO2(g)��H2(g)����Ӧ��CO�����ʵ����ڲ�ͬ�¶�ʱ���ʵ�����ʱ��ı仯���±���ʾ���ش��������⣺
CO2(g)��H2(g)����Ӧ��CO�����ʵ����ڲ�ͬ�¶�ʱ���ʵ�����ʱ��ı仯���±���ʾ���ش��������⣺
 2SO3��״̬��ʱ��ƽ�⣬��O2��ת����Ϊ�� ��
2SO3��״̬��ʱ��ƽ�⣬��O2��ת����Ϊ�� ��