��Ŀ����
����Ŀ���������£����й��ڵ���ʵ�˵������ȷ����
A. �к͵��������Ũ�ȵİ�ˮ������������Һ��pH��7��ǰ�����ĵ������
B. ��NH4Cl��Һ�м���������Ũ�ȵ�ϡ���ᣬ��![]() ��ֵ��С
��ֵ��С
C. ��NaHS��Һ�м������� KOH ��c(Na��)��c(H2S)��c(HS��)��c(S2��)
D. ��a mol��L��1�Ĵ�����0.01 mol��L��1������������Һ��������(����������¶ȱ仯)��������Һ��c(Na��)��c(CH3COO��)�������ĵ��볣��Ka��![]() (�ú�a�Ĵ���ʽ��ʾ)
(�ú�a�Ĵ���ʽ��ʾ)
���𰸡�C
���������к͵��������Ũ�ȵİ�ˮ������������Һ��pH��7����ˮ���ĵ������٣���A������NH4Cl��Һ�м���������Ũ�ȵ�ϡ���ᣬ��������笠�����ˮ�⣬����![]() ��ֵ����B���������������غ㣬��NaHS��Һ�м������� KOH ��c(Na��)��c(H2S)��c(HS��)��c(S2��)����C��ȷ����a mol��L��1�Ĵ�����0.01 mol��L��1������������Һ��������(����������¶ȱ仯)��������Һ��c(Na��)��c(CH3COO��)=0.005 mol��L��1����c(CH3COOH)=
��ֵ����B���������������غ㣬��NaHS��Һ�м������� KOH ��c(Na��)��c(H2S)��c(HS��)��c(S2��)����C��ȷ����a mol��L��1�Ĵ�����0.01 mol��L��1������������Һ��������(����������¶ȱ仯)��������Һ��c(Na��)��c(CH3COO��)=0.005 mol��L��1����c(CH3COOH)= ![]() mol��L��1�������ĵ��볣��Ka��
mol��L��1�������ĵ��볣��Ka��
![]() ����D������
����D������

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�����Ŀ��һ���¶��£���10 mL 0.40 mol��L��1 H2O2��Һ�м�������FeCl3��Һ����ͬʱ�̲������O2�����(������Ϊ��״��)���±���ʾ��
t / min | 0 | 2 | 4 | 6 |
V(O2) / mL | 0 | 9.9 | 17.2 | 22.4 |
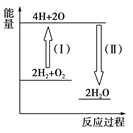
������ʾ����Ӧ���������У��� 2Fe3+��H2O2 == 2Fe2+��O2����2H+���� H2O2��2Fe2+��2H+ == 2H2O��2Fe3+����Ӧ�����������仯����ͼ��ʾ������˵���������
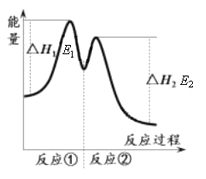
A. Fe3+�������������������ķֽ�����
B. ��Ӧ�������ȷ�Ӧ����Ӧ���Ƿ��ȷ�Ӧ
C. ��Ӧ2H2O2(aq) == 2H2O(l)��O2(g)����H��E1��E2��0
D. 0��6 min��ƽ����Ӧ���ʣ�v(H2O2)��3.33��10��2 mol��L��1��min��1