��Ŀ����
����Ŀ��ʯ���ѽ�����Ҫ���б�ϩ��1,3-����ϩ�Ȳ���������������Ϊԭ�Ͽɺϳ�CR��ҽҩ�м���G���ϳ�·�����£�
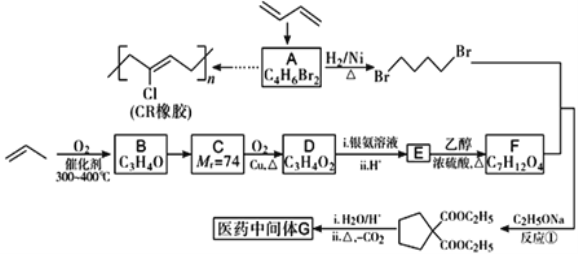
��֪����B��C��D ���ܷ���������Ӧ��
��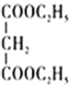
![]()
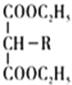
![]() RCH2COOH
RCH2COOH
(1)A��˳ʽ�칹��Ľṹ��ʽΪ_______��
(2)C�к��������ŵ�������________����Ӧ���ķ�Ӧ����Ϊ_______��
(3)д��E��F��Ӧ�Ļ�ѧ����ʽ��_________��
(4)д��ͬʱ��������������ҽҩ�м���G��ͬ���칹��Ľṹ��ʽ�� _______��
����D ��Ϊͬϵ� ���˴Ź�������������塣
(5)�ü�Ҫ���Ա�������B�����������ŵ�ʵ�鷽����_______��
(6)��AΪ��ʼԭ�Ϻϳ�CR����·Ϊ______(�����Լ���ѡ)��
���𰸡�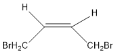 �ǻ���ȩ�� ȡ����Ӧ HOOCCH2COOH+2C2H5OH
�ǻ���ȩ�� ȡ����Ӧ HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O OHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һ��ɫ��֤������̼̼˫��
C2H5OOCCH2COOC2H5+2H2O OHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һ��ɫ��֤������̼̼˫�� ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��������
����A�������ӳɲ������ʽ֪��A�ṹ��ʽΪBrCH2CH��CHCH2Br����ϩ������������Ӧ����B��B�����Ͷȣ�![]() ��2����B�ܷ���������Ӧ��˵������ȩ������B�к���̼̼˫����B�ṹ��ʽΪCH2��CHCHO��B����Է�������Ϊ56��C��B��Է���������18����C�ܷ���������Ӧ����ȩ������B��ˮ�����ӳɷ�Ӧ����C��D����������ӦȻ���ữ����E��E���Ҵ�����������Ӧ����F��F������֪�ڵķ�Ӧ�����������֪��EΪ��Ԫ�ᣬ����C�д��ǻ�λ�ڶ˵㣬��C�ṹ��ʽΪHOCH2CH2CHO��DΪOHCCH2CHO��EΪHOOCCH2COOH��FΪCH3CH2OOCCH2COOCH2CH3��������Ϣ��Ӧ֪��G�ṹ��ʽΪ
��2����B�ܷ���������Ӧ��˵������ȩ������B�к���̼̼˫����B�ṹ��ʽΪCH2��CHCHO��B����Է�������Ϊ56��C��B��Է���������18����C�ܷ���������Ӧ����ȩ������B��ˮ�����ӳɷ�Ӧ����C��D����������ӦȻ���ữ����E��E���Ҵ�����������Ӧ����F��F������֪�ڵķ�Ӧ�����������֪��EΪ��Ԫ�ᣬ����C�д��ǻ�λ�ڶ˵㣬��C�ṹ��ʽΪHOCH2CH2CHO��DΪOHCCH2CHO��EΪHOOCCH2COOH��FΪCH3CH2OOCCH2COOCH2CH3��������Ϣ��Ӧ֪��G�ṹ��ʽΪ![]() ��
��
(6)BrCH2CH��CHCH2Br��NaOH��ˮ��Һ���ȷ���ȡ����Ӧ����HOCH2CH��CHCH2OH��HOCH2CH��CHCH2OH��HCl�����ӳɷ�Ӧ����HOCH2CHClCH2CH2OH��HOCH2CHClCH2CH2OH������ȥ��Ӧ����CH2��CHClCH��CH2��CH2��CHClCH��CH2�����Ӿ۷�Ӧ����CR��
(1)A��˳ʽ�칹��Ľṹ��ʽΪ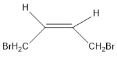 ���ʴ�Ϊ��
���ʴ�Ϊ��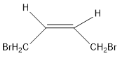 ��
��
(2)C�ṹ��ʽΪHOCH2CH2CHO��C�к��������ŵ�����Ϊ�ǻ���ȩ������Ӧ�ٵķ�Ӧ����ȡ����Ӧ��
�ʴ�Ϊ���ǻ���ȩ����ȡ����Ӧ��
(3)Eת����FΪ��������Ҵ���������Ӧ����Ӧ����ʽΪHOOCCH2COOH+2C2H5OHimg src="http://thumb.zyjl.cn/questionBank/Upload/2020/07/21/23/187b9b2b/SYS202007212347026224939359_DA/SYS202007212347026224939359_DA.002.png" width="97" height="27" style="-aw-left-pos:0pt; -aw-rel-hpos:column; -aw-rel-vpos:paragraph; -aw-top-pos:0pt; -aw-wrap-type:inline" />C2H5OOCCH2COOC2H5+2H2O��
�ʴ�Ϊ��HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O��
C2H5OOCCH2COOC2H5+2H2O��
(4)GΪ![]() ��G��ͬ���칹�������������������D ��Ϊͬϵ�˵����������ȩ���� �ں˴Ź�������������壬˵������3����ԭ�ӣ������������ͬ���칹��Ľṹ��ʽΪOHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO��
��G��ͬ���칹�������������������D ��Ϊͬϵ�˵����������ȩ���� �ں˴Ź�������������壬˵������3����ԭ�ӣ������������ͬ���칹��Ľṹ��ʽΪOHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO��
�ʴ�Ϊ��OHC(CH2)4CHO��OHCCH(CH3)CH(CH3)CHO��
(5)B�ṹ��ʽΪCH2��CHCHO�����к���̼̼˫����ȩ����̼̼˫����������Ȼ�̼��Һ���飬����������ȩ����ȩ��������������Ӧ���飬Ӧ���ȼ���ȩ�����ų�ȩ�����ź��ټ���̼̼˫��������鷽��Ϊ��ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һ��ɫ��֤������̼̼˫����
�ʴ�Ϊ��ȡ����B�ڽྻ�Թ��У���������������Һ��ˮԡ�������������ɣ�֤��B����ȩ�����ټ����ữ����������������Ȼ�̼��Һ����Һ��ɫ��֤������̼̼˫����
(6)BrCH2CH��CHCH2Br��NaOH��ˮ��Һ���ȷ���ȡ����Ӧ����HOCH2CH��CHCH2OH��HOCH2CH��CHCH2OH��HCl�����ӳɷ�Ӧ����HOCH2CHClCH2CH2OH��HOCH2CHClCH2CH2OH������ȥ��Ӧ����CH2��CHClCH��CH2��CH2��CHClCH��CH2�����Ӿ۷�Ӧ����CR����ϳ�·��Ϊ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��
�ʴ�Ϊ��![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() ��
��

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�