��Ŀ����
9��Ϊ��С������Ⱦ�����ܼ�������ҵ�岻�ݴǵ����Σ���1��SO2��Cl2�dz�������Ⱦ�S��Ԫ�����ڱ��е�λ��Ϊ�������ڵڢ�A�壬Cl2�ĵ���ʽΪ
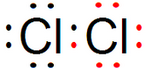 ���Ƚ�S2-��Cl-�İ뾶��С��r��S2-�����������������r��Cl-����
���Ƚ�S2-��Cl-�İ뾶��С��r��S2-�����������������r��Cl-������2����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ��������ܵ���C������ĸ��
A�����ˮ���⣺2H2O$\frac{\underline{\;����\;}}{\;}$2H2��+O2��
B������ʹˮ�ֽ����⣺2H2O$\frac{\underline{\;����\;}}{\;}$2H2��+O2��
C��̫������ֽ�ˮ���⣺2H2O$\frac{\underline{\;\;\;TiO_{2}\;\;\;}}{̫����}$2H2��+O2��
D����Ȼ�����⣺CH4+H2O$\stackrel{����}{?}$CO+3H2
��3���������ϰ�װ��Ч��ת��������ʹ����β���е���Ҫ��Ⱦ�COxNO���������Ӧ�������������ʣ���������β����Ⱦ����֪��
N2��g��+O2��g���T2NO��g����H=+180.5kJ/mol
CO��g��+$\frac{1}{2}$O2��g���TCO2��g����H=-283.0kJ•mol-1
д��β��ת����Ӧ���Ȼ�ѧ��ʽ��2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.5KJ•mol-1��
��4����������������ָ�ڼ�����������Cl2����ˮ�е�NaCN�������������ʣ�NaCN��������IJ���֮һΪNaHCO3��д���÷�Ӧ�����ӷ���ʽ��5Cl2+2CN-+10OH-=2HCO3-+N2+10Cl-+4H2O��
���� ��1��S��16��Ԫ�أ��������Ӳ㣬�������6�����ӣ�������Ԫ�����ڱ��е�λ��Ϊ�������ڣ��ڢ�A����ԭ�������7�����ӣ�����һ�����γ�һ�����õ��ӶԴ�8���ӵ��ȶ��ṹ������Cl2�ĵ���ʽΪ��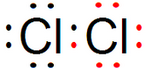 �����Ӳ�ṹ��ͬ�˵����Խ��뾶Խ��
�����Ӳ�ṹ��ͬ�˵����Խ��뾶Խ��
��2���ӳ������̫���ܵĽǶȷ�����
��3���ɢ�N2��g��+O2��g���T2NO��g����H=+180.5kJ/mol����CO��g��+$\frac{1}{2}$O2��g���TCO2��g����H=-283.0kJ•mol-1�����ݸ�˹����2��-�������㻯ѧ��Ӧ���ʱ䣬�Ӷ���д�Ȼ�ѧ����ʽ��
��4����NaCN��������IJ���֮һΪNaHCO3��̼�Ļ��ϼ۸ߣ�˵��NaCN�ǻ�ԭ���������������������ݵ�ʧ�����غ㣬��ƽ��ѧ����ʽ��
��� �⣺��1��S��16��Ԫ�أ��������Ӳ㣬�������6�����ӣ�������Ԫ�����ڱ��е�λ��Ϊ�������ڣ��ڢ�A����ԭ�������7�����ӣ�����һ�����γ�һ�����õ��ӶԴ�8���ӵ��ȶ��ṹ������Cl2�ĵ���ʽΪ��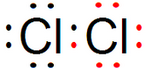 �����Ӳ�ṹ��ͬ�˵����Խ��뾶Խ���ȵĺ˵�������������������ӵİ뾶���������ӣ��ʴ�Ϊ���������ڵڢ�A�壻
�����Ӳ�ṹ��ͬ�˵����Խ��뾶Խ���ȵĺ˵�������������������ӵİ뾶���������ӣ��ʴ�Ϊ���������ڵڢ�A�壻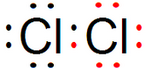 ������
������
��2�����ˮ�������Լ���Ȼ����ʹ�ö���������Դ�������ϵ�̼Ҫ��ʹ��̫���ܿɼ�С��̼���ʴ�Ϊ��C��
��3���ɢ�N2��g��+O2��g���T2NO��g����H=+180.5kJ/mol����CO��g��+$\frac{1}{2}$O2��g���TCO2��g����H=-283.0kJ•mol-1�����ݸ�˹����2��-�ٵ�2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.5KJ•mol-1���ʴ�Ϊ��2NO��g��+2CO��g��=N2��g��+2CO2��g����H=-746.5KJ•mol-1��
��4����NaCN��������IJ���֮һΪNaHCO3��̼�Ļ��ϼ۸ߣ�˵��NaCN�ǻ�ԭ���������������������ݵ�ʧ�����غ㣬���Է�Ӧ����ʽ��5Cl2+2CN-+10OH-=2HCO3-+N2+10Cl-+4H2O���ʴ�Ϊ��5Cl2+2CN-+10OH-=2HCO3-+N2+10Cl-+4H2O��
���� ���⿼��ʹ�û�ʯȼ�ϵ���������Դ�Ŀ������ø�˹���ɽ����йط�Ӧ�ȵļ��㣬����������ԭ��ʵ���ǽ���Ĺؼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | C2H4��C6H6 | B�� | CH4��CH2O | C�� | C2H6��CH3CHO | D�� | C3H8��CH3CH2OH |
| A�� | ��������������ʽ�������ֳ�������������������������������� | |
| B�� | ���ݷ�Ӧ���Ƿ��е���ת�ƽ���ѧ��Ӧ��Ϊ������ԭ��Ӧ�ͷ�������ԭ��Ӧ | |
| C�� | �����Ƿ��ж��������ɢϵ��Ϊ��Һ���������Һ | |
| D�� | �������Ԫ�ص����ཫ�������Ϊ���ʺͻ����� |
| A�� | ��״����6.72 L CO | B�� | 0.3NA��Na2SO3 | ||
| C�� | ���³�ѹ��9.6g O2 | D�� | 1.806��1023��H2O���� |
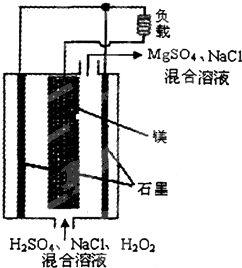 þ-��������ȼ�ϵ�ؾ��б������ߡ���ȫ������ŵ㣬��ṹʾ����ͼ��ʾ�����ڸõ�ص�������ȷ���ǣ�������
þ-��������ȼ�ϵ�ؾ��б������ߡ���ȫ������ŵ㣬��ṹʾ����ͼ��ʾ�����ڸõ�ص�������ȷ���ǣ�������| A�� | ��ع���ʱ��H+�������ƶ� | |
| B�� | �õ�ؿ����ڸ������������� | |
| C�� | ��ع���ʱ��������Χ��Һ��pH�����ϱ�С | |
| D�� | �õ�ص��ܷ�ӦʽΪ��Mg+H2O2+H2SO4�TMgSO4+2H2O |
| A�� | 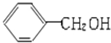 �� ��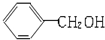 ��Ϊͬϵ�� ��Ϊͬϵ�� | B�� | �Ҵ��Ͷ����ѻ�Ϊͬ���칹�� | ||
| C�� | �Ҵ����Ҷ�������������Ϊͬϵ�� | D�� | ����Ũ��ˮ�������Ҵ��ͱ��� |
| A�� | ��������ӵĵ���ʽ��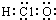 | B�� | CH4Si�Ľṹʽ�� | ||
| C�� | HCN���ӵĽṹʽ��H-C��N | D�� | ������ӵı���ģ�ͣ�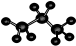 |
| A�� | 0.1mol | B�� | 0.15mol | C�� | 0.5mol | D�� | 0.75mol |