��Ŀ����
�ô����˫��ˮ��Ͽ���������Һ��ϴ�Ӽ�(2Na2CO3��3H2O2)��������ɱ������ȥ���۵������Ҳ�����ȾˮԴ��
(1)������������ϴ�Ӽ��н��������ӵIJ�����������_______________________________________��
(2)����ϴ�Ӽ��е�˫��ˮ���Խ���ˮ�е��軯��ת��Ϊ����ͬʱ����NH3��д����Ӧ�����ӷ���ʽ��___________________________________
(3)�������ϴ�Ӽ���ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��(�����ӷ���ʽ�ͼ�Ҫ���ֱ���)��__________________________________________________________________
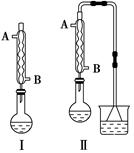
(4)ij��ѧѧϰС��Ϊ����̽�������Ӷ���������ϴ�Ӽ��IJ���Ӱ�죬ȡ��ϴ�Ӽ�100 mL������25 gFeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0.10 mol��L��1 NaOH��Һ��8.0 mol��L��1 NaOH��Һ������ʯ��ˮ��0.01 mol��L��1 KMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��Сľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2��
����2��������________________��
����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۡ�
(1)������������ϴ�Ӽ��н��������ӵIJ�����������_______________________________________��
(2)����ϴ�Ӽ��е�˫��ˮ���Խ���ˮ�е��軯��ת��Ϊ����ͬʱ����NH3��д����Ӧ�����ӷ���ʽ��___________________________________
(3)�������ϴ�Ӽ���ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��(�����ӷ���ʽ�ͼ�Ҫ���ֱ���)��__________________________________________________________________
(4)ij��ѧѧϰС��Ϊ����̽�������Ӷ���������ϴ�Ӽ��IJ���Ӱ�죬ȡ��ϴ�Ӽ�100 mL������25 gFeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0.10 mol��L��1 NaOH��Һ��8.0 mol��L��1 NaOH��Һ������ʯ��ˮ��0.01 mol��L��1 KMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��Сľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2��
����2��������________________��
����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��________��________��ϴ��ƿ�У�________ | __________________ |
��(1)�ýྻ�IJ�˿պȡϴ�Ӽ��ھƾ��ƻ��������գ�����ʻ�ɫ(��������)
(2)H2O2��CN����H2O=HCO3����NH3
(3)2H2O2 2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������
2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������
(4)��CO2��O2
��
(2)H2O2��CN����H2O=HCO3����NH3
(3)2H2O2
 2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������
2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������(4)��CO2��O2
��
| ʵ�鲽�� | Ԥ����������� |
| ����ʯ��ˮ 8.0 mol��L��1 NaOH��Һ�����������ǵ�Сľ���������һ��ϴ��ƿ�ij��ڴ� | ������ʯ��ˮ������ǣ�ľ����ȼ�������1������������ʯ��ˮ����ǣ�ľ����ȼ�������2������������ʯ��ˮ����ǣ�ľ������ȼ�������3���� |
��(1)��������Һ��ϴ�Ӽ���������ΪNa����������ɫ��Ӧ���м��顣(3)�����ӿ�����Ϊ��������ֽ�Ĵ������Ӷ����¹�������ֽ��ʧЧ����������̼������ӷ���ˮ����ٽ���Ӧ��ʹϴ�Ӽ�ʧȥȥ��������(4)�ٸ���ɫ��ζ����������������Ƕ�����̼��������������Ļ�����塣���ó���ʯ��ˮ���������̼����NaOH��Һ����δ��Ӧ��Ķ�����̼�����ͨ�������ǵ�Сľ������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 Fe(SCN)3+3KCl��Һƽ����ϵ�м�����������KCl
Fe(SCN)3+3KCl��Һƽ����ϵ�м�����������KCl

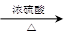 CH3CH2CHCH2����H2O
CH3CH2CHCH2����H2O