��Ŀ����
����Ŀ���̷��е����ʺ����IJⶨ�������á����϶�����������ԭ����ʳƷ������ʹ���һͬ���ȣ�ʹ�����ʷֽ⣬�ֽ�İ�����������������李�Ȼ������ʹ�����룬���������պ�����������������Һ�ζ�������������������Ի���ϵ������Ϊ�����ʺ�����
�������裺
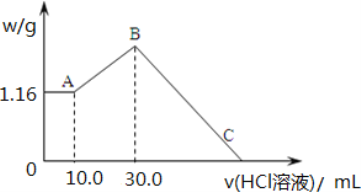
����Ʒ������ȷ��ȡһ�����Ĺ�����Ʒ�̷ۣ����������ձ��У�����һϵ�еĴ���������ȴ������һ�����������ƿ�С�
��NH3����������գ����Ƶõ���Һ(ȡһ����)��ͨ������װ�ã�����һϵ�еķ�Ӧ��ʹ���������泥��پ�������������Ϊ����̬�����백���������ա�
�۰��ĵζ����ñ�������Һ�ζ������ɵ�����泥������ĵ������Һ������ܵ�����������Ϊ�ֵ�������
��ش��������⣺
��1������Ʒ�Ĵ���������ʹ�õ�������ƿ�������������ƿ�Ƿ�©ˮ_________��
��2�������ƹ����У����������������ʹ���Ƶ���Һ��Ũ��ƫ��_______��
A���ձ�����Һת�Ƶ�����ƿ��ʱ��δϴ���ձ� B������ʱ�����ӿ̶���
C������ʱ�����ӿ̶��� D����Һʱ��������Һ�彦��
��3������ȡ��Ʒ������Ϊ1.5g��������100mL����Һ��ȡ���е�20mL������һϵ�д�����ʹNת��Ϊ�����Ȼ����0.1mol��L��1����ζ�������ȥ��������Ϊ23.0mL�������Ʒ��N�ĺ���Ϊ________��
(�ζ��������漰���ķ�Ӧ����ʽ��(NH4)2B4O7��2HCl��5H2O=2NH4Cl��4H3BO3)
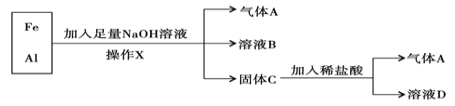
��4��һЩ������ũ���á����϶�������ֻ��Ԫ�صĺ������ó������ʵĺ��������ⷨ��ȱ�㣬�Ա�ţ�̼��ʱ�����ʵĺ�����꣬����ţ�������������谷(C3N6H6)���������谷�е��ĺ���Ϊ______��
���𰸡�������ƿ��ע��һ������ˮ������ƿ������ת�������۲��Ƿ�©ˮ��Ȼ�������ţ���תƿ��180�㣬�ٵ�ת�������۲��Ƿ�©ˮ��������©ˮ����˵��������ƿ��©ˮ B10.73%66.7%
��������
(1)A��������Һʱ���ձ�����Һת�Ƶ�����ƿ��ʱ��δϴ���ձ�������������ʧ��ʹ��ҺŨ��ƫС����A����B������ʱ�����ӿ̶��ߣ�������Һ�����С��ʹ��ҺŨ��ƫ��B��ȷ��C������ʱ�����ӿ̶��ߣ�������Һ�������ʹ��ҺŨ��ƫС����C����D�����ں������ʵ�Һ������ʹ���ʵ����ʵ�����С��������ҺŨ��ƫС����D������ȷ��ΪB��
(2) �ζ�ʱ������������ʵ���n(HCl)= 0.1 mol/L��0.023L=0.0023mol������ʾ�ķ�Ӧ����ʽ�ɵ�n(NH4+)= n(N)= n(HCl)=0.0023mol��������Ʒ��N�ĺ���Ϊ= ![]() ��100%=10.73%��
��100%=10.73%��

����Ŀ��������X����Y��Һ�У����ɳ������ʵ���n2������X�����ʵ���n1�Ĺ�ϵ��ͼ��ʾ������ͼ��ʾ�������
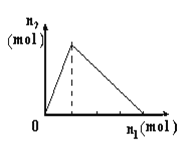
A | B | C | D | |
X | NaOH | AlCl3 | HCl | NaAlO2 |
Y | AlCl3 | NaOH | NaAlO2 | HCl |
A. A B. B C. C D. D
����Ŀ���״�����Ҫ�Ļ�������ԭ�Ϻ����Һ��ȼ�ϣ���ҵ�Ͽ�����CO��CO2�������״�����֪�Ʊ��״����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ�����±���ʾ:
��ѧ��Ӧ | ƽ�ⳣ�� | �¶�/�� | |
500 | 800 | ||
��2H2(g)+CO(g) | K1 | 2.5 | 0.15 |
��H2(g) +CO2 (g) | K2 | 1.0 | 2.50 |
��3H2(g)+CO2(g) | K3 | ||
(1)�ں����ܱ������з�����Ӧ�ڣ��ﵽƽ��������¶ȣ�����˵����ȷ����_______��
a.ƽ�������ƶ� b.��������ƽ����Է����������� c.CO2��ת��������
(2)K1��K2��K3�Ĺ�ϵ��:K3=_______��
(3)500��ʱ��÷�Ӧ��ijʱ�̣�H2(g)��CO2(g)��CH3OH(g)H2O(g)��Ũ��(mol/L)�ֱ�Ϊ0.2��0.1��0.01��0.2�����ʱV��____V��(�>����=����<��)��
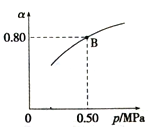
(4)ij�¶��·�Ӧ����H2��ƽ��ת����(a)����ϵ��ѹǿ(P)�Ĺ�ϵ��ͼ��ʾ������ʼ����2mol/LH2��1mol./LCO����B��ʱ��ѧƽ�ⳣ��Ϊ___________��
(5)��ͬ�¶��£��ڼס��������ݻ���ȵĺ����ܱ������У�Ͷ��H2��CO2��������Ӧ������ʼŨ�����±���ʾ�����м�2min��ƽ�⣬ƽ��ʱc(H2O)=0.05mol/L������CO2��ת����Ϊ_______������CO2��ת����____�ס�(��� �ڡ������� �ڡ���С�ڡ�)
��ʼŨ�� | �� | �� |
c(H2)/mol/L | 0.10 | 0.20 |
c(CO2 )/mol/L 0.10 | 0.20 |