ЬтФПФкШн
ЁОЬтФПЁПЯжгаЪвЮТЯТШмжЪХЈЖШОљЮЊ![]() ЕФМИжжШмвКЃКЂйбЮЫсЁЂЂкСђЫсЁЂЂлДзЫсЁЂЂмСђЫсяЇЁЂЂнАБЫЎЁЂЂоЧтбѕЛЏФЦШмвКЃЌЛиД№ЯТСаЮЪЬтЃК
ЕФМИжжШмвКЃКЂйбЮЫсЁЂЂкСђЫсЁЂЂлДзЫсЁЂЂмСђЫсяЇЁЂЂнАБЫЎЁЂЂоЧтбѕЛЏФЦШмвКЃЌЛиД№ЯТСаЮЪЬтЃК
(1)дкЂмШмвКжаЃЌИїРызгХЈЖШДѓаЁЫГађЮЊЃК____________
(2)НЋЂлЁЂЂоЛьКЯКѓЃЌШєШмвКГЪЯжжаадЃЌдђЯћКФСПШмвКЕФЬхЛ§ЮЊЂл____________Ђо(ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБ)ЃЌШмвКжаЕФРызгХЈЖШгЩДѓЕНаЁЕФЫГађЮЊ____________
(3)дкГЃЮТЯТЃЌНЋ100mLЕФЂкгы100mLЕФЂоШмвКЛьКЯКѓ(МйЩшЛьКЯКѓШмвКЕФЬхЛ§ЮЊЛьКЯЧАШмвКЕФЬхЛ§жЎКЭ)ЃЌШмвКЕФpH=____________(вбжЊ![]() )
)
(4)дкГЃЮТЯТЃЌСљжжвКЬхЕФpHгЩДѓЕНаЁЕФЫГађЪЧ____________
(5)ШєНЋЂлШмвККЭЂоШмвКАДЬхЛ§БШ2:1ЛьКЯКѓШмвКГЪЫсадЃЌдђЛьКЯКѓШмвКжа![]() __________
__________![]() (ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБ)
(ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБ)
(6)ГЃЮТЯТНЋЂлШмвКМгЫЎЯЁЪЭЙ§ГЬжаЃЌЯТСаБэДяЪНЕФЪ§ОнвЛЖЈБфДѓЕФЪЧ______
AЁЂ![]() BЁЂ
BЁЂ![]() CЁЂ
CЁЂ![]() DЁЂ
DЁЂ![]()
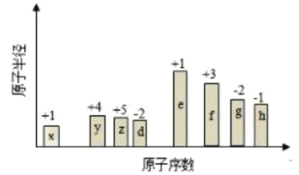
ЁОД№АИЁПc(NH4ЃЋ)>c(SO42Ѓ)>c(HЃЋ)>c(OHЃ) > c(NaЃЋ)=c(CH3COOЃ)>c(HЃЋ)=c(OHЃ) 3.3 Ђо>Ђн>Ђм>Ђл>Ђй>Ђк > B D
ЁОНтЮіЁП
(1)ЂмЮЊ(NH4)2SO4ЃЌ![]() ЫЎНтШмвКГЪЫсадЃЌЕЋЫЎНтЪЧЮЂШѕЕФЃЌВЛЛсДяЕН50%ЃЌШмвКжаc(
ЫЎНтШмвКГЪЫсадЃЌЕЋЫЎНтЪЧЮЂШѕЕФЃЌВЛЛсДяЕН50%ЃЌШмвКжаc(![]() )ЃОc(
)ЃОc(![]() )ЃЌc(HЃЋ)ЃОc(OH)ЃЌЫљвдРызгХЈЖШДѓаЁЮЊЃКc(
)ЃЌc(HЃЋ)ЃОc(OH)ЃЌЫљвдРызгХЈЖШДѓаЁЮЊЃКc(![]() )ЃОc(
)ЃОc(![]() )ЃОc(HЃЋ)ЃОc(OH)ЃЌ
)ЃОc(HЃЋ)ЃОc(OH)ЃЌ
ЙЪД№АИЮЊЃКc(![]() )ЃОc(
)ЃОc(![]() )ЃОc(HЃЋ)ЃОc(OH)ЃЛ
)ЃОc(HЃЋ)ЃОc(OH)ЃЛ
(2)ЂлЮЊCH3COOHЃЌЂоЮЊNaOHЃЌШєЧЁКУЗДгІЩњГЩCH3COONaЃЌCH3COONaЫЎНтШмвКЮЊМюадЃЌЮЊЪЙШмвКГЪзнжаадашвЊЩдЙ§СПЕФCH3COOHЃЌЫљвдЯћКФСПШмвКЕФЬхЛ§ЮЊЂлЃОЂоЃЌШмвКЮЊжаадЃЌc(HЃЋ)ЃНc(OH)ЃЌИљОнЕчКЩЪиКуЃКc(NaЃЋ)ЃЋc(HЃЋ)ЃНc(OH)ЃЋc(CH3COO)ЃЌЫљвдc(NaЃЋ)ЃНc(CH3COO)ЃЌдђШмвКжаРызгХЈЖШДѓаЁЙиЯЕЮЊЃКc(NaЃЋ)ЃНc(CH3COO)ЃОc(HЃЋ)ЃНc(OH)ЃЌ
ЙЪД№АИЮЊЃКЃОЃЛc(NaЃЋ)ЃНc(CH3COO)ЃОc(HЃЋ)ЃНc(OH)ЃЛ
(3)ГЃЮТЯТЃЌНЋ100mLЕФЂкгы100mLЕФЂоШмвКЛьКЯЃЌЗЂЩњЗДгІЃКH2SO4ЃЋ2NaOHЈTNa2SO4ЃЋ2H2OЃЌЕШЬхЛ§ЕШХЈЖШЗДгІЃЌЫсЙ§СПЃЌдђЗДгІКѓШмвКжаc(HЃЋ)ЃН![]()
mol/LЃН5ЁС104mol/LЃЌдђШмвКpHЃНlgc(HЃЋ)ЃН3.3ЃЌ
ЙЪД№АИЮЊЃК3.3ЃЛ
(4)СђЫсЮЊЖўдЊЫсЃЌЭЌХЈЖШЕФЬѕМўЯТЃЌСђЫсЫсадЧПгкбЮЫсЃЌДзЫсЮЊШѕЕчНтжЪЃЌЫсадШѕгкбЮЫсЃЌСђЫсяЇЫЎНтЮЊЫсадЃЌЫсадИќШѕЃЌАБЫЎЮЊШѕМюЃЌЧтбѕЛЏФЦЮЊЧПМюЃЌЫсаддНШѕЃЌpHжЕдНДѓЃЌЫљвдЮхжжвКЬхЕФpHгЩДѓЕНаЁЕФЫГађЪЧЃКЂо>Ђн>Ђм>Ђл>Ђй>ЂкЃЌ
ЙЪД№АИЮЊЃКЂо>Ђн>Ђм>Ђл>Ђй>ЂкЃЛ
(5)НЋЂлШмвККЭЂоШмвКАДЬхЛ§БШ2ЃК1ЛьКЯКѓШмвКГЪЫсадЃЌЯрЕБгкЕШСПЕФCH3COOHКЭCH3COONaЕФЬѕМўЯТШмвКЮЊЫсадЃЌдђДзЫсЕФЕчРыГЬЖШДѓгкЫЎНтГЬЖШЃЌдђЛьКЯКѓШмвКжаc(CH3COO)ЃОc(CH3COOH)ЃЌ
ЙЪД№АИЮЊЃКЃОЃЛ
(6)AЃЎЯЁЪЭЙ§ГЬжаЃЌn(HЃЋ)діДѓЃЌЕЋШмвКЬхЛ§діМгИќДѓЃЌећЬхРДЫЕc(HЃЋ)МѕаЁЃЌЙЪAВЛбЁЃЛ
BЃЎЯЁЪЭЙ§ГЬжаc(HЃЋ)МѕаЁЃЌдђc(OH)ЃН![]() діДѓЃЌЙЪBбЁЃЛ
діДѓЃЌЙЪBбЁЃЛ
CЃЎKwЃН![]() жЛЫцЮТЖШИФБфЖјИФБфЃЌЙЪCВЛбЁЃЛ
жЛЫцЮТЖШИФБфЖјИФБфЃЌЙЪCВЛбЁЃЛ
D.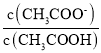 ЃЌЯЁЪЭЙ§ГЬжаc(HЃЋ)МѕаЁЃЌдђ
ЃЌЯЁЪЭЙ§ГЬжаc(HЃЋ)МѕаЁЃЌдђ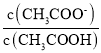 діДѓЃЌЙЪDбЁЃЌ
діДѓЃЌЙЪDбЁЃЌ
ЙЪД№АИЮЊЃКBDЁЃ

ЁОЬтФПЁПФмдДЪЧЙњУёОМУЗЂеЙЕФживЊЛљДЁЃЌЮвЙњФПЧАЪЙгУЕФФмдДжївЊЪЧЛЏЪЏШМСЯЁЃ
(1)вбжЊЃК ![]()
![]()
![]()
дђУКЦјЛЏжївЊЗДгІ![]() ________________
________________
(2)вбжЊ![]() ЕФе§ЗДгІЫйТЪЮЊ
ЕФе§ЗДгІЫйТЪЮЊ![]() ЃЌФцЗДгІЫйТЪЮЊ
ЃЌФцЗДгІЫйТЪЮЊ![]() ЃЌkЮЊЫйТЪГЃЪ§ЁЃ2500KЪБЃЌ
ЃЌkЮЊЫйТЪГЃЪ§ЁЃ2500KЪБЃЌ ![]() ЃЌдђИУЮТЖШЯТЕФЗДгІЦНКтГЃЪ§K=_________________ ЁЃ
ЃЌдђИУЮТЖШЯТЕФЗДгІЦНКтГЃЪ§K=_________________ ЁЃ
(3)МзДМжЦМзУбЕФгаЙиЗДгІЮЊЃК ![]() вЛЖЈЮТЖШЯТЃЌдкШ§ИіШнЛ§ОљЮЊ1.0 LЕФКуШнУмБеШнЦїжаЗЂЩњИУЗДгІЁЃ
вЛЖЈЮТЖШЯТЃЌдкШ§ИіШнЛ§ОљЮЊ1.0 LЕФКуШнУмБеШнЦїжаЗЂЩњИУЗДгІЁЃ
ШнЦїБрКХ | ЮТЖШЃЏЁц | Ц№ЪМЮяжЪЕФСПЃЏmol | ЦНКтЮяжЪЕФСП/mol | |
CH3OH | CH3OCH3 | H2O | ||
I | 387 | 0. 20 | x | |
II | 387 | 0. 40 | y | |
Ђѓ | 207 | 0. 20 | 0. 090 | 0. 090 |
![]() ________________.
________________.
ЂквбжЊ![]() ЪБИУЗДгІЕФЛЏбЇЦНКтГЃЪ§K=4ЁЃИУЮТЖШЯТЃЌШєЦ№ЪМЪБЯђШнЦїIжаГфШы0.10mol
ЪБИУЗДгІЕФЛЏбЇЦНКтГЃЪ§K=4ЁЃИУЮТЖШЯТЃЌШєЦ№ЪМЪБЯђШнЦїIжаГфШы0.10mol ![]() ЃЌдђЗДгІНЋЯђ_________ЃЈЬюЁАе§ЁБЛђЁАФцЁБЃЉЗДгІЗНЯђНјааЁЃ
ЃЌдђЗДгІНЋЯђ_________ЃЈЬюЁАе§ЁБЛђЁАФцЁБЃЉЗДгІЗНЯђНјааЁЃ
ЂлШнЦїЂђжаЗДгІДяЕНЦНКтКѓЃЌШєвЊНјвЛВНЬсИпМзУбЕФВњТЪЃЌПЩвдВЩШЁЕФДыЪЉЮЊ______________ЃЈЬюађКХЃЉ
A.Щ§ИпЮТЖШ B.ЦфЫћЬѕМўВЛБфЃЌдіМг![]() ЕФЮяжЪЕФСП
ЕФЮяжЪЕФСП
C.НЕЕЭЮТЖШ D.БЃГжЦфЫћЬѕМўВЛБфЃЌЭЈШыФЪЦј
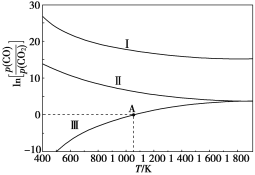
(4)ЮЊМѕЩйЮэіВЁЂНЕЕЭДѓЦјжагаКІЦјЬхКЌСПЃЌбаОПЛњЖЏГЕЮВЦјжа![]() МА
МА![]() ЕФХХЗХСПвтвхжиДѓЁЃЛњЖЏГЕЮВЦјЮлШОЮяЕФКЌСПгыПеЃЏШМБШЃЈПеЦјгыШМгЭЦјЕФЬхЛ§БШЃЉЕФБфЛЏЙиЯЕЪОвтЭМШчЭМЫљЪОЃК
ЕФХХЗХСПвтвхжиДѓЁЃЛњЖЏГЕЮВЦјЮлШОЮяЕФКЌСПгыПеЃЏШМБШЃЈПеЦјгыШМгЭЦјЕФЬхЛ§БШЃЉЕФБфЛЏЙиЯЕЪОвтЭМШчЭМЫљЪОЃК

ЂйЕБПеЃЏШМБШДяЕН15Кѓ![]() МѕЩйЕФдвђПЩФмЪЧ__________ЃЈЬюзжФИЃЉЁЃ
МѕЩйЕФдвђПЩФмЪЧ__________ЃЈЬюзжФИЃЉЁЃ
a.ЗДгІ![]() ЪЧЮќШШЗДгІ
ЪЧЮќШШЗДгІ
b.ЕБПеЃЏШМБШДѓИЩ15КѓЃЌШМгЭЦјШМЩеЗХГіЕФШШСПЯргІМѕЩй
ЂкЫцПеЃЏШМБШдіДѓЃЌCOКЭ![]() ЕФКЌСПМѕЩйЕФдвђЪЧ______ЁЃ
ЕФКЌСПМѕЩйЕФдвђЪЧ______ЁЃ