��Ŀ����
����Ŀ����֪�л�����һ��̼ԭ�������������ǻ�ʱ������ˮ�γ�̼��˫��������A��F������ת����ϵ��

�Իش��������⣺
��1��E�к��еĹ����ŵ�������________��C�����Ƶ�������ͭ����Һ��Ӧ�Ļ�ѧ����ʽΪ________��
��2��A��NaOH��Һ����ʱ��Ӧ�Ļ�ѧ����ʽΪ________��
��3����֪B����Է�������Ϊ162����ȼ�ղ�����n(CO2)��n(H2O)��2��1����B�ķ���ʽΪ________��
��4��F���������ص㣺���ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ����F�����ϵ�һ�ȴ���ֻ�����֣�F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ________��
��5��������G��F��ͬ���칹�壬�����ڷ����廯����ܷ���������Ӧ����G������________�ֽṹ
���𰸡�
��1���Ȼ���CH3CHO+2Cu(OH)2+NaOH![]() CH3COONa+Cu2O��+3H2O��
CH3COONa+Cu2O��+3H2O��
��2�� CH3CHBrOOCCH3��2NaOH![]() CH3CHO��CH3COONa��H2O��NaBr��
CH3CHO��CH3COONa��H2O��NaBr��
��3��C10H10O2��
��4��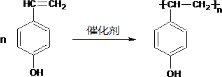 ��
��
��5��4��![]() ��
��![]() ��
��![]() ��
��![]() ������2�֡�
������2�֡�
��������
���������A����ˮ�ⷴӦC��D��D�ữ�õ�E��C�����õ�E����C�Ľṹ��ʽΪCH3CHO��DΪCH3COONa��EΪCH3COOH��B����Է�������Ϊ162������ȫȼ�յIJ�����n(CO2):n (H2O)=2:1����B��̼��ԭ�Ӹ���֮��Ϊ1:1������a=b���������Է�������֪��a=b=![]() =10������B�ķ���ʽΪC10H10O2��Bˮ������E(����)��F��F�ķ���ʽΪC10H10O2+H2O-C2H4O2=C8H8O�������Ͷ�Ϊ5��F���������ص������ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ�������ϵ�һ�ȴ���ֻ����������F���з��ǻ�������1��-CH=CH2����2��ȡ�������ڶ�λ����F�Ľṹ��ʽΪ��
=10������B�ķ���ʽΪC10H10O2��Bˮ������E(����)��F��F�ķ���ʽΪC10H10O2+H2O-C2H4O2=C8H8O�������Ͷ�Ϊ5��F���������ص������ܸ�FeCl3��Һ������ɫ��Ӧ�����ܷ����Ӿ۷�Ӧ�������ϵ�һ�ȴ���ֻ����������F���з��ǻ�������1��-CH=CH2����2��ȡ�������ڶ�λ����F�Ľṹ��ʽΪ��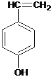 ��BΪ
��BΪ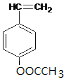 ��
��
��1��������������֪��EΪCH3COOH�����еĹ����������Ȼ���CΪ��ȩ��������������ͭ��Ӧ����ʽΪ��CH3CHO+2Cu(OH)2+NaOH![]() CH3COONa+Cu2O��+3H2O���ʴ�Ϊ���Ȼ���CH3CHO+2Cu(OH)2+NaOH
CH3COONa+Cu2O��+3H2O���ʴ�Ϊ���Ȼ���CH3CHO+2Cu(OH)2+NaOH![]() CH3COONa+Cu2O��+3H2O��
CH3COONa+Cu2O��+3H2O��
��2��A��NaOH��Һ���ȷ���ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪCH3CHBrOOCCH3��2NaOH![]() CH3CHO��CH3COONa��H2O��NaBr���ʴ�Ϊ��CH3CHBrOOCCH3��2NaOH
CH3CHO��CH3COONa��H2O��NaBr���ʴ�Ϊ��CH3CHBrOOCCH3��2NaOH![]() CH3CHO��CH3COONa��H2O��NaBr��
CH3CHO��CH3COONa��H2O��NaBr��
��3��������������֪��B�ķ���ʽΪC10H10O2���ʴ�Ϊ��C10H10O2��
��4��������������֪��FΪ ����һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ��
����һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��������G��F��ͬ���칹�壬G���ڷ����廯����ܷ���������Ӧ��G�к���ȩ�������ܵ�ͬ���칹����![]() ��
��![]() ��
��![]() ��
��![]() ��4�֣��ʴ�Ϊ��4��
��4�֣��ʴ�Ϊ��4��![]() ��
��![]() ��
��![]() ��
��![]() ������2�֡�
������2�֡�

 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�����Ŀ��ֻ�������м����ж�Ӧ��������������������ʵ��������� ��
A | B | C | D | |
�� | �����е������� | ��״���µ�����Ħ����� | �������� | ��Һ�����ʵ����ʵ���Ũ�� |
�� | �����ӵ����� | ��״���µ��������� | ������ܶ� | ��Һ��� |
����Ŀ��ij�о���С��̽�����������ķ�Ӧ������ʵ�����£�
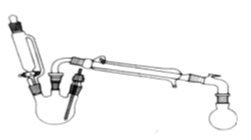
CH3COOH + C2H5OH ![]() CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
Ͷ�� 1 �� 1 CH3COOHת���� 65%
1 �� 10 CH3COOHת���� 97%
(��120 ���²ⶨ)
��֪������������ʣ����³�ѹ��
�ܶ�g/mL | �۵�/�� | �е�/�� | ˮ���� | |
�Ҵ� | 0.79 | -114 | 78 | �� |
���� | 1.049 | 16.2 | 117 | �� |
�������� | 0.902 | 84 | 76.5 | ���� |
�ϳɷ�Ӧ��
������ƿ�м����Ҵ�5 mL������5 mL��2СƬ���Ƭ��©����������14.3 mL ���Ҵ�20 mL����������ͨ����ȴˮ��ʼ�������ȣ����Ƶμ��ٶȵ��������ٶȣ���Ӧ�¶Ȳ�����120 �档
�����ᴿ��
����Ӧ�ֲ��ﵹ���Һ©���У��������������͵�Na2CO3��Һ������NaCl��Һ������CaCl2��Һϴ�ӣ�����������ˮ̼��أ�����һ��ʱ�����ȥ̼��ء�����ͨ������õ�����������������
�ش��������⣺
��1��������Ӧ�Ļ���
���Ҵ��ǻ���ʾ��
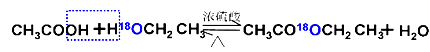
�ô����ǻ���ʾ��

����18ˮռ����ˮ����һ�룬��Ҳһ�������ʵ���Ʒ���������ӦΪ��ȡ����Ӧ����������������Ӧ�Ļ��� ��
2��������Ӧ��һ������ķ�Ӧ��120 ��ʱ��ƽ�ⳣ��K= ��
��3���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������________(����ȷ�𰸱��)��
A���������� B����ȴ�� C�����貹�� D����������
��4��Ũ�������Ҵ���λ�ϣ� ��
��5�����Ƶμ�������Ҵ����Һ���ٶȵ��������ٶ�Ŀ���ǣ� ��
��6�������Ĵ�������������Ҫ����Щ���ʣ� �����͵�Na2CO3��Һϴ�ӳ�ȥ���ᡣ����ж��Ƿ������ ���ñ���NaCl��Һϴ�ӳ�ȥ������Na2CO3��Һ��Ϊʲô����ˮ�� ��