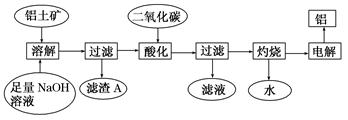
��Ŀ����
��6�֣�һ���Ȼ�þ����������ɵĻ�����У�Ϊ�ⶨ�����Ȼ�þ�ĵ�����������С��ͬѧ��ȡ�û������Ʒ20g����ȫ����ˮ�У�Ȼ��ȡ����һ��������������������������Һ100g��ƽ�����Ĵμ������У�������ʵ���������±���ʾ���������������صļ��㣺
���ϱ���X����ֵ�� ��
������ԭ����������Ʒ���Ȼ�þ������������ ��
| ���� | 1 | 2 | 3 | 4 |
| ��������������Һ��������g�� | 25 | 25 | 25 | 25 |
| ���ɳ�����������g�� | 2.9 | X | 8.7 | 8.7 |
������ԭ����������Ʒ���Ȼ�þ������������ ��
�� 5.8��2�֣��� �� 71.25%
��1������ʵ��1��3��֪��25g����������Һ������2.9g������������ʵ��2��Ӧ������5.8g������
��2������ʵ��3��4��֪�����������ֵ��8.7g����������þ��8.7g�����Ը��ݷ���ʽ2NaOH��MgCl2=Mg(OH)2����2NaCl��֪���Ȼ�þ��������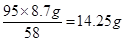 ������ԭ����������Ʒ���Ȼ�þ������������
������ԭ����������Ʒ���Ȼ�þ������������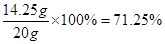 ��
��
��2������ʵ��3��4��֪�����������ֵ��8.7g����������þ��8.7g�����Ը��ݷ���ʽ2NaOH��MgCl2=Mg(OH)2����2NaCl��֪���Ȼ�þ��������
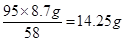 ������ԭ����������Ʒ���Ȼ�þ������������
������ԭ����������Ʒ���Ȼ�þ������������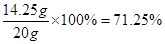 ��
��
��ϰ��ϵ�д�
�����Ŀ
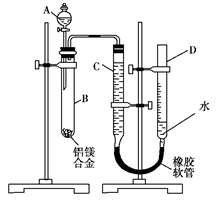
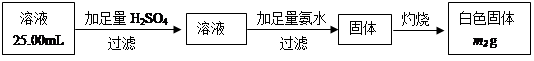
 �����������������ml��������ɱ�״������
�����������������ml��������ɱ�״������ ���ʣ����������a�ˣ�
���ʣ����������a�ˣ�  ��ó���������b�ˡ�
��ó���������b�ˡ�