��Ŀ����
(16��)ij�о�С��ȡ������״����������ͬ����Ƭ���ֱ���Ũ�Ⱦ�Ϊ6 mol?L��1�ĵ���������ᡢ����������Һ��ַ�Ӧ��ȡ��ʣ�����Ƭϴ�������������
��1��������Ӧʣ����Ƭ��������ǰ��________���ߣ�����ڡ���С�ڡ����ڡ�)��
��2���۲���Ƭ�����ᷴӦ�����Һ�ϻ��ǣ����Թܵײ���������ɫ���塣
��������֪��
���������ᷴӦ����Ӧ�������ɷ�ĩ��ɢ����Һ�У��γɺ�ɫ����
�ڵ�ⷨұ�������õ�ԭ���������Ǵ�����������ȡ�ģ������������������������������Ͷ����������ʡ�д���ڵ��������ɵ��������ʶ������跴Ӧ�Ļ�ѧ����ʽ�� ________��
��3��Ϊ̽����2���к�ɫ����ijɷ֣�����������衣
����1����ɫ������Al��Fe
����2����ɫ������Al��Si
����3����ɫ������Al��_______________
��4�����ʵ�鷽�������ڼ���3����ʵ�飬��֤��ɫ���庬��Al֮��������ɷ֡�
��ѡʵ���Լ�������ˮ��6mol��L-1H2SO4��Һ��6mol��L-1NaOH��Һ��6mol��L-1��ˮ��0.01mol��L-1KMnO4��Һ��������ˮ��20%KSCN��Һ��
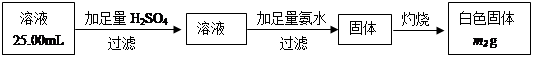
��5����֪����NaOH��Һ��Ӧ�������ơ�ȡm1g��ɫ���������NaOH��Һ����ܽ⣬
��ȥ����������ǿ��������ʧ�������250mL��Һ��ȡ��25.00mL��Һ�������в�������ʵ�飺
�����������У���Ҫ�õ��IJ�������������________________________________��
�����ɫ������Al����������Ϊ__________________�����ԭ��������Al-27 Fe-56 H-1 O-16����
��1��������Ӧʣ����Ƭ��������ǰ��________���ߣ�����ڡ���С�ڡ����ڡ�)��
��2���۲���Ƭ�����ᷴӦ�����Һ�ϻ��ǣ����Թܵײ���������ɫ���塣
��������֪��
���������ᷴӦ����Ӧ�������ɷ�ĩ��ɢ����Һ�У��γɺ�ɫ����
�ڵ�ⷨұ�������õ�ԭ���������Ǵ�����������ȡ�ģ������������������������������Ͷ����������ʡ�д���ڵ��������ɵ��������ʶ������跴Ӧ�Ļ�ѧ����ʽ�� ________��
��3��Ϊ̽����2���к�ɫ����ijɷ֣�����������衣
����1����ɫ������Al��Fe
����2����ɫ������Al��Si
����3����ɫ������Al��_______________
��4�����ʵ�鷽�������ڼ���3����ʵ�飬��֤��ɫ���庬��Al֮��������ɷ֡�
��ѡʵ���Լ�������ˮ��6mol��L-1H2SO4��Һ��6mol��L-1NaOH��Һ��6mol��L-1��ˮ��0.01mol��L-1KMnO4��Һ��������ˮ��20%KSCN��Һ��
| ʵ�鲽�� | Ԥ��ʵ������ͽ��� |
| ����1������Ӧ������Һ���ˡ�ϴ�ӣ�ȡ�����������Թ��У�����������6mol��L-1 H2SO4��Һ����������á� | ___________________________________�� ֤����ɫ���庬��Si |
| ����2�� | |
��ȥ����������ǿ��������ʧ�������250mL��Һ��ȡ��25.00mL��Һ�������в�������ʵ�飺

�����������У���Ҫ�õ��IJ�������������________________________________��
�����ɫ������Al����������Ϊ__________________�����ԭ��������Al-27 Fe-56 H-1 O-16����
��1�����ڣ�2�֣� ��2����4Al��3SiO23Si��2Al2O3��3�֣� ��3��Fe��Si��2�֣�
��4��
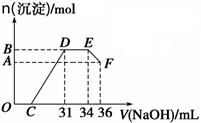
��5��250mL����ƿ����ʽ�ζ��ܣ�2�֣� 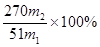 ��3�֣�
��3�֣�
��4��
| ����1�� | ������ų�����ɫ���岿���ܽ⣨1�֣� |
| ����2��ȡA�Թ��������ϲ���ҹ����������������ˮ���ٵμ�1~2��20%KSCN��Һ��2�֣� �������������𰸣� | ��Һ�Ժ�ɫ��֤����ɫ���庬��Fe��1�֣� |
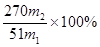 ��3�֣�
��3�֣���1�������������ĵ���������������Ƶģ�����������ʣ������ࡣ
��2�����Ͷ�������ķ�Ӧ���û���Ӧ����ӦʽΪ4Al��3SiO23Si��2Al2O3��
��3�����ݼ���1��2��֪������3Ӧ��Al��Fe��Si��
��4���������ܺ��ᷴӦ���費�ܡ������ܺ�����������Һ��Ӧ���������ܣ��ݴ˿��Լ���
��5������һ�����ʵ���Ũ����Һ��Ҫ����ƿ����Һ�Լ��ԣ�ȷ��ȡ��Һ��Ҫ��ʽ�ζ��ܡ����õ��Ĺ���������������ɫ������Al����������Ϊ
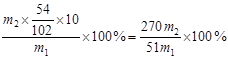
��2�����Ͷ�������ķ�Ӧ���û���Ӧ����ӦʽΪ4Al��3SiO23Si��2Al2O3��
��3�����ݼ���1��2��֪������3Ӧ��Al��Fe��Si��
��4���������ܺ��ᷴӦ���費�ܡ������ܺ�����������Һ��Ӧ���������ܣ��ݴ˿��Լ���
��5������һ�����ʵ���Ũ����Һ��Ҫ����ƿ����Һ�Լ��ԣ�ȷ��ȡ��Һ��Ҫ��ʽ�ζ��ܡ����õ��Ĺ���������������ɫ������Al����������Ϊ
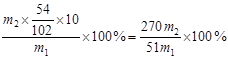

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ