��Ŀ����
����Ŀ����.���Ĺ̶��Ǽ���������ѧ��һֱ�о��Ŀ��⡣
��1���±��о��˲�ͬ�¶��´����̵���ҵ�̵��IJ��ֻ�ѧƽ�ⳣ��K��ֵ��
��Ӧ | �����̵�N2(g)��O2(g)2NO(g) | ��ҵ�̵�N2(g)��3H2(g)2NH3(g) | |||
�¶�/�� | 27 | 2 000 | 25 | 400 | 450 |
ƽ�ⳣ��K | 3.84��10��31 | 0.1 | 5��108 | 0.507 | 0.152 |
�ٷ������ݿ�֪�������̵���Ӧ����________(����ȡ����ȡ�)��Ӧ��
�ڷ������ݿ�֪������ʺϴ��ģģ������̵���ԭ��____________________________��
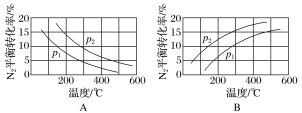
��2����ҵ�̵���Ӧ�У�������������ͬʱ���ֱ�ⶨN2��ƽ��ת�����ڲ�ͬѹǿ(p1��p2)�����¶ȱ仯�����ߣ���ͼ��ʾ��ͼʾ�У���ȷ����________(�A����B��)���Ƚ�p1��p2�Ĵ�С��ϵ��________��
��.Ŀǰ��ҵ�ϳɰ���ԭ����N2(g)��3H2(g)2NH3(g)��
��3����һ���¶��£���1 mol N2��3 mol H2����������������ܱ������з�����Ӧ���ﵽƽ��״̬ʱ��������������ʵ���Ϊ2.8 mol��
�ٴ�ƽ��ʱ��H2��ת���ʦ�1��________��
����֪ƽ��ʱ������ѹǿΪ8 MPa����ƽ�ⳣKp��________(��ƽ���ѹ����Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
���𰸡�����KֵС��������еij̶�С(��ת���ʵ�)�����ʺϴ��ģ����Ap2��p160%49/192��0.255��0.26
��������
��. ��1�����ɱ������ݿ�֪���¶�Խ�ߣ�KԽ��˵�������¶ȣ�ƽ�����ƣ�������Ӧ����Ϊ���ȷ�Ӧ��
���ɱ������ݿ�֪��2000��ʱ��K=0.1��Kֵ��С����ת���ʺ�С�����ʺϴ��ģ��������������ʺϴ��ģģ������̵���
��2���ϳɰ���ӦΪ���ȷ�Ӧ�������¶ȣ�ת���ʼ�С������ͼA��ȷ��B����ѡA���÷�Ӧ������Ϊ�����С�ķ�������ѹǿƽ�������ƶ���ת����������2��ת���ʴ�����2����2>��1��
���� ��3������ƽ��ʱ��x molN2ת����
N2��g��+3H2��g��2NH3��g��
��ʼ���ʵ����� 1mol 3mol 0
�仯�����ʵ�����x 3x 2x
ƽ�����ʵ�����1-x 3-3x 2x
��ʽ�ɵã���1-x��+��3-3x��+2x=2.8�����x=0.6mol����1 =![]() ��100%=60%��
��100%=60%��
��ƽ��ʱ�����ʵ�ѹǿ֮�ȵ��������ʵ���֮�ȣ�����P��N2��=![]() ��8MPa=
��8MPa=![]() MPa��P��H2��=
MPa��P��H2��=![]() ��8MPa=
��8MPa=![]() MPa��P��NH3��=
MPa��P��NH3��=![]() ��8MPa=
��8MPa=![]() MPa����ѧƽ�ⳣ��Kp=
MPa����ѧƽ�ⳣ��Kp=![]() =
=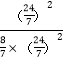 ��0.26��
��0.26��

 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�