��Ŀ����
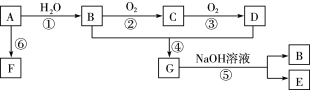
����Ŀ������֥�Ӹ��ѧ��ѧ�о������ս�ʾ��WO3/CeO2-TiO2˫�����ڵ����´�������Ⱦ������ת��Ϊ�����壬�������ͼ��ʾ
(1)Ti�۵����Ų�ʽΪ______________����������ߵ��ܲ���___________��
(2)N��O��H����ԭ�ӵĵ縺���ɴ�С��˳��Ϊ___________________��
(3)NO2-�Ŀռ乹��Ϊ___________����NO3-��Ϊ�ȵ�����ķ���Ϊ_______________
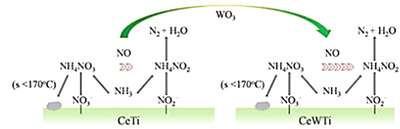
(4)WO3����Ϊ����ϩ�����Ĵ���![]() +H2O2
+H2O2![]()
![]() +H2O
+H2O
��![]() ��̼ԭ�ӵ��ӻ���ʽΪ_________��
��̼ԭ�ӵ��ӻ���ʽΪ_________��
��1molH2O2����������Ϊ____________��
��![]() ����______________���塣
����______________���塣
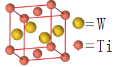
(5)W��Ti���γɽ��������ijW��Ti�Ľ�����������ͼ��ʾ����þ���Ļ�ѧʽΪ__________��
(6)�����ѵľ���ѻ���ʽΪ ____________
���𰸡�3d24s2 N O>N>H V�� SO3 sp2 3NA ���� WTi ��TiW �������ܶѻ�
��������
(1)Ti��ԭ������Ϊ22���õ��۵����Ų�ʽ��������ߵ��ܲ㡣
(2)ͬ���ڣ������ң��縺��������
(3)NO2����N�۲���Ӷ�Ϊ3�ԣ�����һ�Թ¶Ե��ӣ��ó��ռ乹�͡����ݼ۵�����S = O = N����д���ȵ����塣
(4)��![]() ��̼ԭ�Ӽ۲���Ӷ�Ϊ3�ԡ�
��̼ԭ�Ӽ۲���Ӷ�Ϊ3�ԡ�
��H2O2�ĽṹʽΪH��O��O��H���ó�1mol H2O2��![]() ����
����
��![]() ���ڷ��Ӿ��塣
���ڷ��Ӿ��塣
(5)�ɾ����ṹ�������֪�������к���2��Ti��2��W��
(6)���䳣�������ľ���ѻ���ʽ��
(1)Ti��ԭ������Ϊ22���۵����Ų�ʽΪ3d24s2�����ڵ������ڣ�������ߵ��ܲ���N�㣬�ʴ�Ϊ��3d24s2��N��
(2)ͬ���ڣ������ң��縺�����������N��O��H����ԭ�ӵĵ縺���ɴ�С��˳��ΪO>N>H���ʴ�Ϊ��O>N>H��
(3)NO2����N�۲���Ӷ�Ϊ3�ԣ�NΪsp2�ӻ�������һ�Թ¶Ե��ӣ��ռ乹��ΪV�Σ�NO3���м۵�����Ϊ24�����ݼ۵�����S = O = N�������NO3����SO3��Ϊ�ȵ����壬�ʴ�Ϊ��V�Σ�SO3��
(4)��![]() ��̼ԭ�Ӽ۲���Ӷ�Ϊ3�ԣ��ӻ���ʽΪsp2���ʴ�Ϊ��sp2��
��̼ԭ�Ӽ۲���Ӷ�Ϊ3�ԣ��ӻ���ʽΪsp2���ʴ�Ϊ��sp2��
��H2O2�ĽṹʽΪH��O��O��H��1mol H2O2��![]() ��Ϊ3NA���ʴ�Ϊ��3NA��
��Ϊ3NA���ʴ�Ϊ��3NA��
��![]() ���ڷ��Ӿ��壬�ʴ�Ϊ�����ӡ�
���ڷ��Ӿ��壬�ʴ�Ϊ�����ӡ�
(5)�ɾ����ṹ��֪�������к���2��Ti��2��W����þ���Ļ�ѧʽΪWTi��TiW���ʴ�Ϊ��WTi��TiW��
(6)�����ѵľ���ѻ���ʽΪ�������ܶѻ����ʴ�Ϊ���������ܶѻ���