��Ŀ����
������ԭ�ζ�ʵ��ͬ�к͵ζ�����(����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮)������0.001 mol��L��1KMnO4������Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ���ӷ���ʽ��2 ��
�� ��H��===2Mn2����5
��H��===2Mn2����5 ��3H2O����ջش����⣺
��3H2O����ջش����⣺
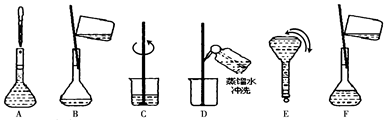
(1)�õζ�ʵ�����������������е�________(�����)��
E������̨ F���ζ��ܼ�
G���ձ� H����ֽ
I����ͷ�ι� J��©��
(2)����________(��ᡱ�)ʽ�ζ���ʢ�Ÿ��������Һ���Է���ԭ��
________________________________________________________________________��
(3)ѡ����ָʾ����˵������
________________________________________________________________________��
(4)�ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL����(b��a)mL��ʵ������KMnO4��Һ���ƫ________(��ࡱ���١�)������(b��a) mL����õ��Ĵ���Ũ�ȣ���ʵ��Ũ��ƫ________(���С��)��
 ��
�� ��H��===2Mn2����5
��H��===2Mn2����5 ��3H2O����ջش����⣺
��3H2O����ջش����⣺(1)�õζ�ʵ�����������������е�________(�����)��
| A����ʽ�ζ���(50 mL) | B����ʽ�ζ���(50 mL) |
| C����Ͳ(10 mL) | D����ƿ |
G���ձ� H����ֽ
I����ͷ�ι� J��©��
(2)����________(��ᡱ�)ʽ�ζ���ʢ�Ÿ��������Һ���Է���ԭ��
________________________________________________________________________��
(3)ѡ����ָʾ����˵������
________________________________________________________________________��
(4)�ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL����(b��a)mL��ʵ������KMnO4��Һ���ƫ________(��ࡱ���١�)������(b��a) mL����õ��Ĵ���Ũ�ȣ���ʵ��Ũ��ƫ________(���С��)��
(1)ABDEFH
(2)����������ǿ�������ܸ�ʴ��
(3)����ָʾ������Ϊ Mn2��ʱ��ɫ��ȥ
Mn2��ʱ��ɫ��ȥ
(4)�١�С
(2)����������ǿ�������ܸ�ʴ��
(3)����ָʾ������Ϊ
 Mn2��ʱ��ɫ��ȥ
Mn2��ʱ��ɫ��ȥ(4)�١�С
���������(1)��Ϊ������ԭ�ζ�ʵ���������к͵ζ������к͵ζ�ʵ������������ѡ�ý���Ǩ�ƿɵó���ȷ�𰸡�
(2)����KMnO4����ǿ�����ԣ��ܸ�ʴ�ܣ��ʲ����ü�ʽ�ζ���ʢ��KMnO4��Һ��
(3)
 Ϊ��ɫ��Mn2��Ϊ��ɫ��������һ���Ե���ɫ�仯���жϵζ��յ㡣
Ϊ��ɫ��Mn2��Ϊ��ɫ��������һ���Ե���ɫ�仯���жϵζ��յ㡣(4)�ζ�����Һ�棬�������ƫС������Ũ�ȱ�ʵ��Ũ��ƫС��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ