��Ŀ����
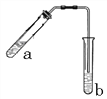
����Ŀ����ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ������ʵ���������������
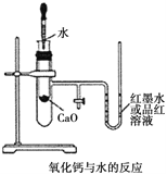
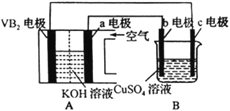
�� ��ͼ��ʾ��ʵ��װ�����Ӻã�
�� �� U �ι��ڼ���������īˮ(��Ʒ����Һ)���� T �ιܻ�����ʹ U �ι������ߵ�Һ�洦��ͬһˮƽ �棬�ٹر� T �ιܻ�����
�� ��ʢ�� 1.0g �����Ƶ�С�Թ������ 2mL ���ҵ�����ˮ���۲����� �Իش�
��1��ʵ��ǰ������е�һ��ʵ�������_____��
��2��ʵ���й۲쵽��������_____��
��3��˵��CaO��H2O��������Ca(OH)2������֮��Ĺ�ϵ��_________��
��4������ʵ���� CaO ���� NaCl��ʵ�黹�ܷ�۲쵽��ͬ����?_____(�������� ��������)��
���𰸡����װ�������� U �ι����Һ������½����ұ����� 1mol CaO �� 1mol H2O �������ʹ��� 1mol Ca(OH)2 ������ ��
��������
(1)��ʵ����������ѹԭ���µ�ʵ��������֣�����ʵ��֮ǰһ��Ҫ���װ�������ԣ��ʴ�Ϊ�����װ�������ԣ�
(2)CaO��ˮ��Ӧ�ų�������ʹ���Թ��п������ͣ��ڲ�ѹǿ������U�ι����Һ������½����ұ��������ʴ�Ϊ��U�ι����Һ������½����ұ�������
(3)CaO+H2O�TCa(OH)2������ʵ������֪���������ƺ�ˮ֮��ķ�Ӧ�Ƿ��ȵģ�1molCaO��1molH2O�������ʹ���1molCa(OH)2���������ʴ�Ϊ��1molCaO��1molH2O�������ʹ���1molCa(OH)2��������
(4)�Ȼ��ƺ�ˮ��Ϻ������ı仯�ܲ����ԣ��Թ�������ѹǿ�������䣬��������κ����ʴ�Ϊ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������й�������������;���ж�Ӧ��ϵ����
ѡ�� | ���� | ���� | ��; |
A | Si | Ӳ�ȴ� | ̫���ܵ�ذ� |
B | NH3 | ��ԭ�� | ����� |
C | HC1O | ǿ������ | Ư�� |
D | Na2O2 | ����ɫ���� | ������ |
A.AB.BC.CD.D