��Ŀ����
12������ʵ���ܴﵽԤ��Ŀ���ǣ�������| A�� | ���Բ��ö���������������ķ������롢�ᴿ������ | |
| B�� | �������Һ�м���ϡH2SO4��ˮԡ����һ��ʱ���ȡ������ˮ��Һ�μӵ�ˮ������Һ������֤������δ����ˮ�� | |
| C�� | �����Ǻ�ϡH2SO4����ˮ����Һ��ȡ���������������Ƶ�Cu��OH��2��������У���ɫ�������ɣ�֤������δ����ˮ������������ | |
| D�� | ��������Һ�м����ͪ����ʹ�����ʴ���Һ���������ټ�ˮ�����ܽ� |
���� A�����������ڽ��壬�����ڱ�������Һ����������Ĥ��
B�������������ɫ��
C�������Ǻ�����������ͭ����Һ�ڼ��������·���������Ӧ��
D�������ʱ���û�п����ԣ�
��� �⣺A�����������ڽ��壬�����ڱ�������Һ����������Ĥ�����ö���������������ķ������롢�ᴿ�����ʣ���A��ȷ��
B�������������ɫ����ʵ���е��۲���ˮ�⡢��ˮ�ⶼ��ʹ��ˮ����ɫ����B����
C�������Ǻ�����������ͭ����Һ�ڼ��������·���������Ӧ�������ڵμ�����������ͭ����Һ֮ǰӦ�ü�NaOH�к��ᣬ��C����
D����ͪ��ʹ�����ʱ��Զ�����Һ���������������ʱ���û�п����ԣ����Լ�ˮ�����ܽ⣬��D����
��ѡA��
���� ���⿼�黯ѧʵ�鷽�����ۣ��漰�����ʡ����ۼ������ŵļ����֪ʶ�㣬��ȷʵ��ԭ���ǽⱾ��ؼ������ʵ������淶�Լ������Է����жϣ��״�ѡ����C��ע�⵰���ʱ��Ժ���������

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ
3��ij�����ӷϺ����л������л������Ĺ���������ͼ��ʾ����֪�ϴ���������Ni70.0%��һ������Al��Fe��SiO2���л�������仯̨��Ļ�ѧ�������������ƣ���Ni2-�����ʽ��ȶ�����

��֪�������������������������ʽ��ȫ����ʱ��pH���±���ʾ���ش����м���
��1������a�ijɷ���SiO2�����Ҵ�ϴ�ӷϴ�����Ŀ�����ܽ⡢��ȥ�л���ӷ����л����Ҵ��ķ���������
��2��Ϊ���������ʣ��ɲ�ȡ�Ĵ�ʩ�У������������ϴ���������ʵ�����������Ũ�ȡ�����ʱ���¶ȣ�
��3����A�м��� H2O2ʱ��Ӧ�����ӷ���ʽΪ2Fe2++2H++H2O2=2Fe3++2H2O���Լ�x������NaOH��
��4���÷���ʽ��ʾ���ɳ�������ȡ�������ķ���Ni��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$NiO+H2O��2Al+2NiO$\frac{\underline{\;����\;}}{\;}$Al2O3+3Ni��
����mkg�����ϴ����Ĺ����У�������ҺpH����Ϊ6ʱ������a kgNi��OH��2����ϴ�ӵ��õ���ҺB�Ĺ�����������ʧ��Ϊ3%��������������������ʧ��Ϊ5%�������յõ�������������Ϊ��70%��97%m+$\frac{59}{93}$a����95%kg�������ʽ����
��5�����û�ѧ�ƣ����Ƽ�ֱ�����ں��жƲ�����Ļ��������Һ�У������ڽ��������ϡ��մɵ���Ʒ�������һ�����������Ƚ�����ij��ѧ��������Һ�к���Ni2+��H2PO2-�������������·����ķ�Ӧ֮һ���£�����ƽ�÷�Ӧ��2Ni2++1H2PO2-+H2O=2Ni++1H2PO3-+2H+
������ȣ���ѧ�Ƶ�����ŵ��ǣ������ĵ��ܣ���Լ��Դ��

��֪�������������������������ʽ��ȫ����ʱ��pH���±���ʾ���ش����м���
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
��2��Ϊ���������ʣ��ɲ�ȡ�Ĵ�ʩ�У������������ϴ���������ʵ�����������Ũ�ȡ�����ʱ���¶ȣ�
��3����A�м��� H2O2ʱ��Ӧ�����ӷ���ʽΪ2Fe2++2H++H2O2=2Fe3++2H2O���Լ�x������NaOH��
��4���÷���ʽ��ʾ���ɳ�������ȡ�������ķ���Ni��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$NiO+H2O��2Al+2NiO$\frac{\underline{\;����\;}}{\;}$Al2O3+3Ni��
����mkg�����ϴ����Ĺ����У�������ҺpH����Ϊ6ʱ������a kgNi��OH��2����ϴ�ӵ��õ���ҺB�Ĺ�����������ʧ��Ϊ3%��������������������ʧ��Ϊ5%�������յõ�������������Ϊ��70%��97%m+$\frac{59}{93}$a����95%kg�������ʽ����
��5�����û�ѧ�ƣ����Ƽ�ֱ�����ں��жƲ�����Ļ��������Һ�У������ڽ��������ϡ��մɵ���Ʒ�������һ�����������Ƚ�����ij��ѧ��������Һ�к���Ni2+��H2PO2-�������������·����ķ�Ӧ֮һ���£�����ƽ�÷�Ӧ��2Ni2++1H2PO2-+H2O=2Ni++1H2PO3-+2H+
������ȣ���ѧ�Ƶ�����ŵ��ǣ������ĵ��ܣ���Լ��Դ��
7�������йػ�ѧʵ��������У���ȷ���ǣ�������
| A�� | �ü�ʽ�ζ�����ȡ20mL0.1000mol/LKMnO4��Һ | |
| B�� | ֻ��Ũ��ˮ�Ϳ��Լ���NaCl��AlCl3��MgCl2��Na2SO4������Һ | |
| C�� | �����Ȼ�̼��ȡ��ˮ�е��岢��Һ���л���Ӧ�ӷ�Һ©�����Ͽڵ��� | |
| D�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У�������¶ȱ仯ֵƫС |
17������������кͷ�Ӧ�Ȼ�ѧ����ʽ���ã�H+��aq��+OH-��aq��H2O��l����H=-57.3kJ•mol-1����ʾ���ǣ�������
| A�� | CH3COOH��aq��+NaOH��aq���TCH3COONa��aq��+H2O��l������H=-Q1 kJ•mol-1 | |
| B�� | $\frac{1}{2}$H2SO4��Ũ��+NaOH��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l������H=-Q2 kJ•mol-1 | |
| C�� | HNO3��aq��+NaOH��aq���TNaNO3��aq��+H2O��l������H=-Q3 kJ•mol-1 | |
| D�� | $\frac{1}{3}$H3PO4��aq��+$\frac{1}{2}$Ba��OH��2��aq���T$\frac{1}{6}$Ba3��PO4��2��s��+H2O��l������H=-Q4 kJ•mol-1 |
1���������ʵĵ���ʽ��д��ȷ���ǣ�������
��Ca��OH��2
 ��H2S��
��H2S��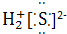
��OH-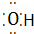 ��Al3+��Al3+ ��N2��N$\underset{\stackrel{•}{•}}{•}$ $\underset{\stackrel{•}{•}}{•}$N
��Al3+��Al3+ ��N2��N$\underset{\stackrel{•}{•}}{•}$ $\underset{\stackrel{•}{•}}{•}$N
��CO2 ��HClO��
��HClO��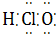 ��Na2O2��
��Na2O2��
��Ca��OH��2

 ��H2S��
��H2S��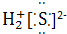
��OH-
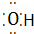 ��Al3+��Al3+ ��N2��N$\underset{\stackrel{•}{•}}{•}$ $\underset{\stackrel{•}{•}}{•}$N
��Al3+��Al3+ ��N2��N$\underset{\stackrel{•}{•}}{•}$ $\underset{\stackrel{•}{•}}{•}$N��CO2
 ��HClO��
��HClO��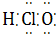 ��Na2O2��
��Na2O2��
| A�� | �٢ڢۢ� | B�� | �ݢޢߢ� | C�� | �ڢۢݢޢ� | D�� | �٢ܢ� |
2������������ȷ���ǣ�������
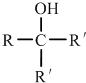
| A�� | ������Ϊ35����ԭ�ӣ�${\;}_{35}^{17}$Cl | B�� | NCl3�ĵ���ʽΪ 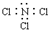 | ||
| C�� | ���������Ľṹ��ʽ��C2H5OOCH | D�� | ������ĽṹʽH-Cl-O |
 ��
��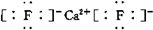 ����XY2Ϊ���ۻ�����ʱ����ṹʽΪ��S=C=S��
����XY2Ϊ���ۻ�����ʱ����ṹʽΪ��S=C=S��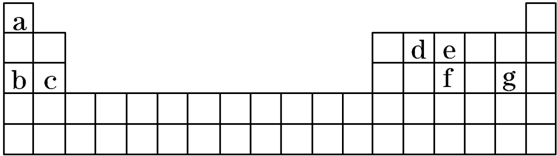
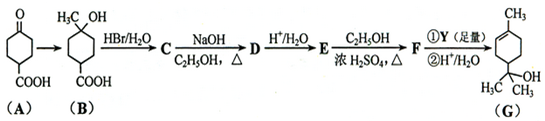
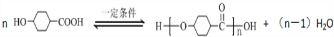
 ���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ
���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ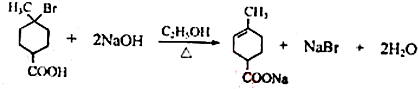 ��
��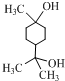 ��
��