��Ŀ����
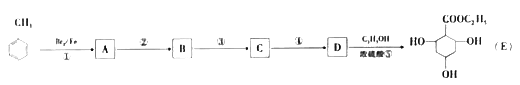
����Ŀ���Ǽ��㶹����һ�����Ƶ���ʯ��ҩ��ϳ�·������ͼ��ʾ��
��֪��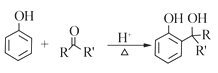
RCOOR'+R'OH![]() RCOOR'+ R'OH��R��R'��R'����������
RCOOR'+ R'OH��R��R'��R'����������
��1��A���ڷ���������ṹ��ʽ��__________________��B�������Ĺ�������_____________��
��2��C��D�ķ�Ӧ������___________________��
��3��E�������ࡣ�����Ҵ�Ϊ�л�ԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�E��д���йػ�ѧ����ʽ��______________________________��
��4����֪��2E![]() F+C2H5OH��F������������
F+C2H5OH��F������������![]() ��___________��
��___________��
��5����D��FΪԭ�Ϻϳ��Ǽ��㶹�ط�Ϊ������Ӧ��д���йػ�����Ľṹ��ʽ��
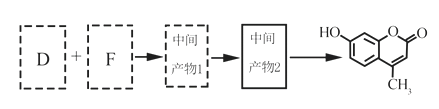 D:______________��F:______________������1:______________������2:______________
D:______________��F:______________������1:______________������2:______________
���𰸡� ![]() ���� ȡ����Ӧ 2CH3CH2OH+O2
���� ȡ����Ӧ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O��2CH3CHO+O2
2CH3CHO+2H2O��2CH3CHO+O2![]() 2CH3COOH��CH3CH2OH��CH3COOH
2CH3COOH��CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O ![]()
 CH3CH2OOCCH2COCH3
CH3CH2OOCCH2COCH3 

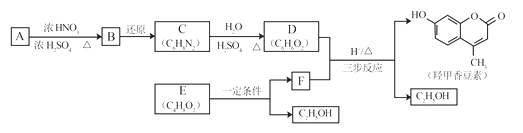
��������(1)A�Ƿ�����������C�Ľṹ��ʽ�Լ�A��B�ķ�Ӧ��������֪A��B�DZ���������Ӧ����֪AΪ�����ṹ��ʽΪ�� ��
��![]() �DZ���������Ӧ����ȡ����Ӧ������������������B�������Ĺ���������������ȷ����
�DZ���������Ӧ����ȡ����Ӧ������������������B�������Ĺ���������������ȷ����![]() �� ������
�� ������
��2��B�е���������ԭΪ��������Ũ������ȵ������£�ˮ�е��ǻ�ȡ�������������ķ�Ӧ��ȡ����Ӧ����ȷ����ȡ����Ӧ��
��3��E�����࣬ԭ�Ͻ�Ϊ�Ҵ�������E�������������ϳɸ�����Ҫ�����Ҵ���������ȩ����ȩ����Ϊ������������Ҵ�����������Ӧ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��2CH3CHO+O2
2CH3CHO+2H2O��2CH3CHO+O2![]() 2CH3COOH��CH3CH2OH��CH3COOH
2CH3COOH��CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O����ȷ����2CH3CH2OH+O2
CH3COOCH2CH3��H2O����ȷ����2CH3CH2OH+O2![]() 2CH3CHO+2H2O��2CH3CHO+O2
2CH3CHO+2H2O��2CH3CHO+O2![]() 2CH3COOH��CH3CH2OH��CH3COOH
2CH3COOH��CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��
��4������ԭ���غ㣬�Ƴ�F�ķ���ʽΪ![]() ��������Ϣ���Լ��Ǽ��㶹�صĽṹ��ʽ���Ƴ�F�Ľṹ��ʽΪ
��������Ϣ���Լ��Ǽ��㶹�صĽṹ��ʽ���Ƴ�F�Ľṹ��ʽΪ �����˺���
�����˺��� ��������
��������![]() ����ȷ����
����ȷ����![]() ��
��
��5�������Ǽ��㶹�صĽṹ��ʽ���Լ���֪��Ϣ���Ƴ�D�Ľṹ��ʽΪ ����������������F�Ľṹ��ʽΪ
����������������F�Ľṹ��ʽΪ ��F��D�������ơ���֪��Ϣ1���з�Ӧ�����м����1��
��F��D�������ơ���֪��Ϣ1���з�Ӧ�����м����1�� ���м����1�ٷ������ơ���֪��Ϣ2���еķ�Ӧ�����м����2��
���м����1�ٷ������ơ���֪��Ϣ2���еķ�Ӧ�����м����2�� ������м����2�еĴ��ǻ�������ȥ��Ӧ�����Ǽ��㶹�أ���ȷ�𰸣�
������м����2�еĴ��ǻ�������ȥ��Ӧ�����Ǽ��㶹�أ���ȷ�𰸣� ��CH3CH2OOCCH2COCH3 ��
��CH3CH2OOCCH2COCH3 ��  ��
��  ��
��