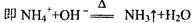��Ŀ����
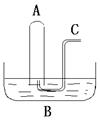
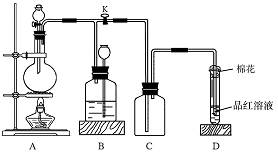
����18�֣�ij��ѧ��ȤС���ڼ��������ö���������Ũ���ᷴӦ����ȡ���ռ�������

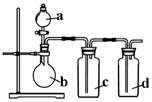
��1����ȡ����ʱ����ƿ�м���һ�����Ķ������̣�ͨ�� �����������ƣ�����ƿ�м���������Ũ���ᣬд���÷�Ӧ�Ļ�ѧ����ʽ ��
��2������װ����ȡ�õ��������п��ܺ������� ����� ���壬��Ҫ�õ����������������Ӧʹ��������ͨ��װ�� �� ��ϴ��ƿ��
��3��������Ӧ�У���������
��4����Ӧ����������������������Ϊ�˷�ֹ��Ⱦ�����������β��ͨ�� �У�д����Ӧ�����ӷ���ʽ
��5����С������200mL 10mol��L-1��Ũ�����������Ķ������̹�����ȷ�Ӧ���������Ȼ��ӷ�������������������ʵ���Ϊ 0��5mol������ڡ��������ڡ���С�ڡ�����ԭ���� ��
��6��87g����������������Ũ���ᷴӦ�������ɵ������ڱ�������Ϊ���٣��μӷ�Ӧ�������б��������Dz��ֵ����ʵ����Ƕ��٣������ԭ��������Mn��55 O��16 Ҫ��д�������ʽ��

��1����ȡ����ʱ����ƿ�м���һ�����Ķ������̣�ͨ�� �����������ƣ�����ƿ�м���������Ũ���ᣬд���÷�Ӧ�Ļ�ѧ����ʽ ��
��2������װ����ȡ�õ��������п��ܺ������� ����� ���壬��Ҫ�õ����������������Ӧʹ��������ͨ��װ�� �� ��ϴ��ƿ��
��3��������Ӧ�У���������
��4����Ӧ����������������������Ϊ�˷�ֹ��Ⱦ�����������β��ͨ�� �У�д����Ӧ�����ӷ���ʽ
��5����С������200mL 10mol��L-1��Ũ�����������Ķ������̹�����ȷ�Ӧ���������Ȼ��ӷ�������������������ʵ���Ϊ 0��5mol������ڡ��������ڡ���С�ڡ�����ԭ���� ��
��6��87g����������������Ũ���ᷴӦ�������ɵ������ڱ�������Ϊ���٣��μӷ�Ӧ�������б��������Dz��ֵ����ʵ����Ƕ��٣������ԭ��������Mn��55 O��16 Ҫ��д�������ʽ��

��

��ϰ��ϵ�д�
�����Ŀ
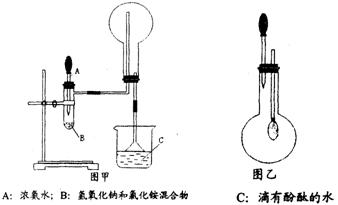
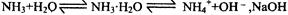 ʹƽ�������ƶ�
ʹƽ�������ƶ�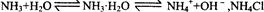 ʹƽ�������ƶ�
ʹƽ�������ƶ�