��Ŀ����
�������������������������Ҫԭ��֮һ��������������ķ����ж��֣�
��1�����ü������ԭ���������֪��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ/mol
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ/mol
��CH4��NO2��ԭΪN2���Ȼ�ѧ����ʽ�� ��
��2������NH3����ԭ�������SCR���������ü�����ĿǰӦ����㷺���������������ѳ������������Ļ�ѧ��Ӧ�ǣ�
2NH3��g��+NO��g��+NO2��g��
2N2��g��+3H2O��g����H��0��
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1��2�ּ��ɣ���
��1�����ü������ԭ���������֪��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ/mol
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ/mol
��CH4��NO2��ԭΪN2���Ȼ�ѧ����ʽ��
��2������NH3����ԭ�������SCR���������ü�����ĿǰӦ����㷺���������������ѳ������������Ļ�ѧ��Ӧ�ǣ�
2NH3��g��+NO��g��+NO2��g��
| 180�� |
| ���� |
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ��
���㣺�Ȼ�ѧ����ʽ,��ѧƽ���Ӱ������
ר�⣺�����������������
��������1�������Ȼ�ѧ����ʽ��˹���ɼ���õ������Ȼ�ѧ����ʽ��
��2����ߵ��������ת���ʣ��ı�����ƽ��������У����ݻ�ѧƽ��Ӱ�����ط����жϣ�
��2����ߵ��������ת���ʣ��ı�����ƽ��������У����ݻ�ѧƽ��Ӱ�����ط����жϣ�
���
�⣺��1����CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ/mol��
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ/mol��
���ݸ�˹����
�õ�CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-867 kJ/mol��
�ʴ�Ϊ��CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-867 kJ/mol��
��2��2NH3��g��+NO��g��+NO2��g��
2N2��g��+3H2O��g����H��0����Ӧ�������������ķ��ȷ�Ӧ�����ݻ�ѧƽ���ƶ�ԭ��������������Ũ�ȣ���Сѹǿ�������¶ȣ�
�ʴ�Ϊ������NH3Ũ�ȣ����Сѹǿ�������¶ȣ���
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ/mol��
���ݸ�˹����
| ��+�� |
| 2 |
�ʴ�Ϊ��CH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��g����H=-867 kJ/mol��
��2��2NH3��g��+NO��g��+NO2��g��
| 180�� |
| ���� |
�ʴ�Ϊ������NH3Ũ�ȣ����Сѹǿ�������¶ȣ���
���������⿼�����Ȼ�ѧ����ʽ��д��˹���ɼ���Ӧ�ã���ѧƽ��Ӱ�����ط����жϣ���Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
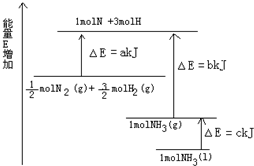
��֪N2��g��+3H2��g��?2NH3��g����H=-92.4kJ?mol-1�����¶���ͬ���ݻ���Ϊ2L��3�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��£���÷�Ӧ�ﵽƽ��ʱ���й��������£�����˵����ȷ���ǣ�������
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1mol N2��3mol H2 | 2mol N2��6mol H2 | 2mol NH3 |
| NH3��Ũ�ȣ�mol?L-1�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�Q1 kJ | �ų�Q2 kJ | ����Q3 kJ |
| ��ϵѹǿ��Pa�� | p1 | p2 | p3 |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
| A��Q3+92.4c1=92.4 |
| B����2+��3��1 |
| C��2p1=2p3��p2 |
| D���ﵽƽ��ʱ��������NH3������������ |
����һ�����ڲ����������ǣ�������
| A��C2H4 |
| B��C4H8 |
| C��C3H8 |
| D��C5H12 |
���������ϵ�����������ͷ�չ�������������DZ��������Լ����ӱ��������ĽǶȳ�����Ŀǰ���з�չǰ����һ���Բ;��ǣ�������
| A�����ʲ;� | B�����ϲ;� |
| C��ֽ�ʲ;� | D������ֲ;� |
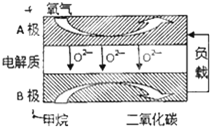
 �����ǵ�ѭ���е���Ҫ���ʣ�����������������������Ź㷺��Ӧ�ã�
�����ǵ�ѭ���е���Ҫ���ʣ�����������������������Ź㷺��Ӧ�ã�