��Ŀ����
A��B��C��D��E������ԭ���������ε����Ķ�����Ԫ�أ���֪������ֻ��һ���ǽ���Ԫ�أ�A��DԪ�ص�ԭ��������������ͬ��C��Eͬ���壬��EԪ��ԭ����������CԪ��ԭ����������2����B�������������ǵ��Ӳ�����������C��A���γ����ֳ�����Һ̬��������ң�����Է��������ұȼ״�16��
��1��EԪ�ص�����Ϊ�� ��D��ԭ�ӽṹʾ��ͼΪ ��
��2�����������к��еĻ�ѧ���� ������Թ��ۼ����Ǽ��Թ��ۼ�������
��3��A������B��C�γɵĻ�����ɺϳ�һ��������������ȼ�ϼ״���
��֪CO��g��+1/2O2��g��=CO2��g��
��H=��283 kJ/mol
H2��g��+1/2O2��g��===H2O��g�� ��H=��242 kJ/mol
CH3OH��g��+3/2O2��g��=CO2��g��+2H2O��g��
��H=��651 kJ/mol
д��A����ѡ������ϳɼ״����Ȼ�ѧ����ʽ�� ��
��4��EC2��C��D�γɵĻ������������ԭ��Ӧ�Ļ�ѧ����ʽΪ�� ��
��1��EԪ�ص�����Ϊ�� ��D��ԭ�ӽṹʾ��ͼΪ ��
��2�����������к��еĻ�ѧ���� ������Թ��ۼ����Ǽ��Թ��ۼ�������
��3��A������B��C�γɵĻ�����ɺϳ�һ��������������ȼ�ϼ״���
��֪CO��g��+1/2O2��g��=CO2��g��
��H=��283 kJ/mol
H2��g��+1/2O2��g��===H2O��g�� ��H=��242 kJ/mol
CH3OH��g��+3/2O2��g��=CO2��g��+2H2O��g��
��H=��651 kJ/mol
д��A����ѡ������ϳɼ״����Ȼ�ѧ����ʽ�� ��
��4��EC2��C��D�γɵĻ������������ԭ��Ӧ�Ļ�ѧ����ʽΪ�� ��
��1���� 
��2�����Թ��ۼ����Ǽ��Թ��ۼ�
��3��CO��g����2H2��g��=CH3OH��g�� ��H=��116 kJ��mol��1
��4��Na2O2��SO2=Na2SO4

��2�����Թ��ۼ����Ǽ��Թ��ۼ�
��3��CO��g����2H2��g��=CH3OH��g�� ��H=��116 kJ��mol��1
��4��Na2O2��SO2=Na2SO4
������Ԫ����ͬ������Ԫ�ص�C��E����Ԫ�أ�E��ԭ��������C��2��,EΪS��CΪO;B�������������ǵ��Ӳ�������������B��ԭ������С��C��BΪ̼Ԫ�أ�A��OԪ���γ����ֳ�����Һ̬�����AΪH��DΪNa����ΪH2O2����ΪH2O��
��1��Na��ԭ�ӽṹʾ��ͼΪ
��2��H2O2�к��еĻ�ѧ��Ϊ���Թ��ۼ����Ǽ��Թ��ۼ���
��3��H2��CO��Ӧ�Ʊ�CH3OH���Ȼ�ѧ����ʽ������
CO��g��+1/2O2��g��=CO2��g�� ��H=��283 kJ/mol ��
H2��g��+1/2O2��g��=H2O��g�� ��H=��242 kJ/mol ��
CH3OH��g��+3/2O2��g��=CO2��g��+2H2O��g�� ��H=��651 kJ/mol �ۿ�֪���٣��ڡ�2���ۼ��ø��Ȼ�ѧ����ʽCO��g����2H2��g��=CH3OH��g�� ��H=��116 kJ��mol��1��
��4��SO2��Na2O2����������ԭ��Ӧ����Na2SO4����Ӧ�Ļ�ѧ����ʽΪNa2O2��SO2=Na2SO4��
��1��Na��ԭ�ӽṹʾ��ͼΪ

��2��H2O2�к��еĻ�ѧ��Ϊ���Թ��ۼ����Ǽ��Թ��ۼ���
��3��H2��CO��Ӧ�Ʊ�CH3OH���Ȼ�ѧ����ʽ������
CO��g��+1/2O2��g��=CO2��g�� ��H=��283 kJ/mol ��
H2��g��+1/2O2��g��=H2O��g�� ��H=��242 kJ/mol ��
CH3OH��g��+3/2O2��g��=CO2��g��+2H2O��g�� ��H=��651 kJ/mol �ۿ�֪���٣��ڡ�2���ۼ��ø��Ȼ�ѧ����ʽCO��g����2H2��g��=CH3OH��g�� ��H=��116 kJ��mol��1��
��4��SO2��Na2O2����������ԭ��Ӧ����Na2SO4����Ӧ�Ļ�ѧ����ʽΪNa2O2��SO2=Na2SO4��

��ϰ��ϵ�д�
�����Ŀ

 >K2��
>K2��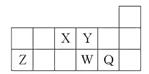

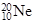 ��
��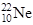 ��˵����ȷ����
��˵����ȷ����