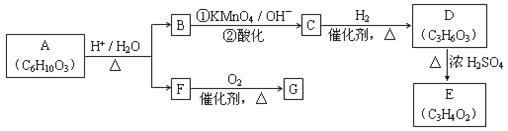
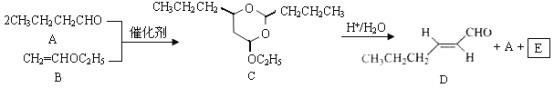
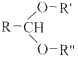
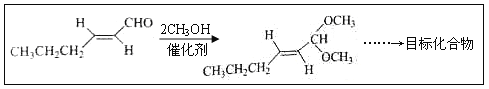
��Ŀ����
����Ŀ��ԭ������С��36��X��Y��Z��W����Ԫ�أ�X��̬ԭ�ӵ����������������ڲ��������2����Y��̬ԭ�ӵ�2pԭ�ӹ������3��δ�ɶԵ��ӣ�Z�ǵؿ��к�������Ԫ�أ�W��ԭ������Ϊ24��
��1��W��̬ԭ�ӵĺ�������Ų�ʽΪ___________��Ԫ��X��Y��Z�ĵ�һ�������ɴ�С��˳��Ϊ___________(��Ԫ�ط��ű���)��
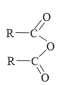
��2����XYZ-��Ϊ�ȵ�����Ļ�ѧʽΪ___________��
��3��1molHYZ3�����к�����������ĿΪ___________��
��4��YH3��������ˮ����Ҫԭ����___________��
���𰸡�1s22s22p63s23p63d54s1 N��O��C CO2��SCN-�ȣ� 4��6.02��1023 ��������ˮ���Ӽ����γ����
��������
ԭ������С��36��X��Y��Z��W����Ԫ�أ�Ԫ��X��ԭ�����������������ڲ��2����Xԭ��ֻ����2�����Ӳ㣬����������Ϊ4����XΪCԪ�أ�Y��̬ԭ�ӵ�2p�������3��δ�ɶԵ��ӣ�Y�ĺ�������Ų�ʽΪ1s22s22p3����YΪNԪ�أ�Z�ǵؿ��к�������Ԫ�أ���ZΪOԪ�أ�W��ԭ������Ϊ24����WΪCrԪ�أ��ݴ˷������
��������������XΪCԪ�أ�YΪNԪ�أ�ZΪOԪ�أ�WΪCrԪ�ء�
(1)W���������Ϊ24����̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1��ͬһ���ڣ���ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���Ԫ��ԭ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�����ܣ�N��O��C���ʴ�Ϊ��1s22s22p63s23p63d54s1��N��O��C��
(2)ԭ��������ȡ��۵��������������Ϊ�ȵ����壬��CNO-��Ϊ�ȵ��������CO2��SCN-�ȣ��ʴ�Ϊ��CO2(��SCN-��)��
(3)HNO3�ĽṹʽΪ![]() ��1molHNO3�����к���4mol��������ĿΪ4��6.02��1023���ʴ�Ϊ��4��6.02��1023��
��1molHNO3�����к���4mol��������ĿΪ4��6.02��1023���ʴ�Ϊ��4��6.02��1023��
(4)��������ˮ���Ӽ����γ����������NH3��������ˮ���ʴ�Ϊ����������ˮ���Ӽ����γ������

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�