��Ŀ����
����Ŀ���о���ѧϰС����Na2SO3��������Һ(������ˮ�����1��1)�Ʊ�SO2����������̽��ʵ�顣

��1��D�е�ʵ��������_____________��C��E����Һ����ɫ���ֱ�����SO2________��_______���ʡ�
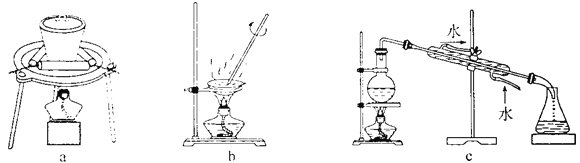
��2�����ڷ����л����ռ�SO2�Լ�β������װ��(������Լ�)��___________
��3���о�С�鷢��B���а�ɫ������Ϊ��������������ʣ��о�С����B�м������ϡ���ᣬ���� ���ܽ⣬����Ϊ���µij�������__________���γɸó����ķ�Ӧ����ʽ�У�_________________________________________________________________________________
Ϊ�˽�һ����֤����ԭ���о�С����ȡBaC12��Һ��������У���ȴ�� ʱ����������Һ�⣬Ȼ����ͨSO2��������ֳ��������٣����������� �ǡ��о�С��Ľ�Aװ�ã���ͼ�����ٽ���ʵ�飬B��û�г��ֻ��ǡ������������ΪX�������_____________

A��CO2 B��NH3 C��O3 D��N2 E��NO2
��4��SO2Ϊ������Ⱦ��о�С��Ϊ�ⶨij������������SO2�ĺ�����ȡ10m3(��״��)�Ŀ����� ����ͨ��������ˮ�У���������Һ�м��������BaC12��Һ���������İ�ɫ����ϴ�ӡ�����õ��ӳƳ�������Ϊ0.233g����
�� ����ϴ���Ѿ��ɾ���ʵ�������_________________________________________________��
�� �Լ���˿�����SO2��Ũ��(�г��������ʽ���������������λ��mg/m3��ʾ)��________________________________________________
���𰸡���1�����ֵ���ɫ����(��ɫ�������е���ɫ�������)(ÿ��2��) Ư���ԡ���ԭ��(��1�֣���2��)
��2�� (2�֣�ֻ���ռ�װ��0�֣�û��ע������ʯ��ˮ��1��)
(2�֣�ֻ���ռ�װ��0�֣�û��ע������ʯ��ˮ��1��)
��3��BaSO4(2��) SO2+H2O![]() H2SO3�� 2H2SO3+O2=2H2SO4�� H2SO4+BaCl2=BaSO4��+2HCl(ÿ��1�֣���3��) AD(2��)
H2SO3�� 2H2SO3+O2=2H2SO4�� H2SO4+BaCl2=BaSO4��+2HCl(ÿ��1�֣���3��) AD(2��)
��4����ȡ���һ��ϴ��Һ�������Թ��У����������ữ��AgNO3��Һ��û����������֤��ϴ�Ӹɾ�(2��)
��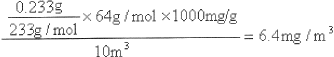 ��2�֣���ʽ1�֣����1�֣�
��2�֣���ʽ1�֣����1�֣�
��������
���⣨1��SO2+2H2S==3S��+2H2O����D�г��ֵ���ɫ����(��ɫ�������е���ɫ�������)��ʹƷ����Һ��ɫ������SO2��Ư���ԣ�ʹ���Ը��������Һ��ɫ������SO2�Ļ�ԭ�ԣ���2��SO2������ˮ���ܶȱȿ������ж�����˳��������ſ��������̳����ռ�����NaOH��Һ���ն����SO2����3��BaSO3��BaSO4��Ϊ�����ǰ�����������ᣬ�������������SO2+H2O![]() H2SO3�� 2H2SO3+O2=2H2SO4�� H2SO4+BaCl2=BaSO4��+2HCl���Ľ���Ŀ���Ƿ�ֹ�����е���������SO2��H2SO3����˳�ͨ��CO2��N2����װ���е���������4���������������ԣ�BaSO4����������BaCl2�����ȡ���һ��ϴ��Һ�������Թ��У����������ữ��AgNO3��Һ��û����������֤��ϴ�Ӹɾ�����m/M��֪��n(BaSO4)=
H2SO3�� 2H2SO3+O2=2H2SO4�� H2SO4+BaCl2=BaSO4��+2HCl���Ľ���Ŀ���Ƿ�ֹ�����е���������SO2��H2SO3����˳�ͨ��CO2��N2����װ���е���������4���������������ԣ�BaSO4����������BaCl2�����ȡ���һ��ϴ��Һ�������Թ��У����������ữ��AgNO3��Һ��û����������֤��ϴ�Ӹɾ�����m/M��֪��n(BaSO4)=![]() ������Ԫ���غ��֪��n(SO2)=n(BaSO4)=
������Ԫ���غ��֪��n(SO2)=n(BaSO4)=![]() ����nM��֪��m(SO2)=
����nM��֪��m(SO2)=![]() ��64gmol��1=
��64gmol��1=![]() ��64gmol��1��1000mg/g�������������SO2��Ũ��Ϊ
��64gmol��1��1000mg/g�������������SO2��Ũ��Ϊ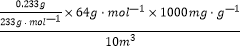 =6.4mgm��3��
=6.4mgm��3��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�