��Ŀ����
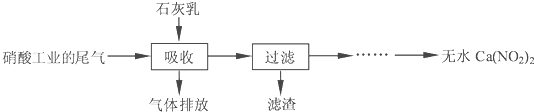
����ʯ��������Ṥҵ��β������NO��NO2����Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca��NO2��2���䲿�ֹ����������£�

��1��һ�������£�NO ��NO2 �������з�Ӧ��NO��g��+NO2��g��?N2O3��g������ƽ�ⳣ������ʽΪK=______��
��2�����������в�����-Һ�����Ӵ����գ�β�������������룬ʯ����������������ܣ�����Ŀ����______��������ѭ��ʹ�ã���������Ҫ�ɷ���______���ѧʽ����
��3���ù��������NO ��NO2 ���ʵ���֮�Ƚӽ�1 ��1����n��NO����n��NO2����1 ��1����ᵼ��______����n��NO����n��NO2����1 ��1����ᵼ��______��
��4����������Һ�豣�������ԣ���������Һ��Ca��NO2��2 �ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ______��
�⣺��1����ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��NO��g��+NO2��g��?N2O3��g������ƽ�ⳣ������ʽΪK= ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��ʹβ����NO��NO2��ʯ�����ֽӴ���NO��NO2��������գ�������Ҫ�ɷ���Ca��OH��2���ʴ�Ϊ��ʹβ����NO��NO2��������գ�Ca��OH��2��
��3����n��NO����n��NO2����1��1����һ�������������ŷ�������NO�������ߣ���n��NO����n��NO2����1��1�����������������������������ʯ���鷴Ӧ����Ca��NO3��2���ʴ�Ϊ���ŷ�������NO�������ߣ���ƷCa��NO2��2��Ca��NO3��2�������ߣ�
��4����Ӧ����NO2-��H+����������һ���������������ˮ����Ӧ�����ӷ���ʽΪ3NO2-+2H+=NO3-+2NO��+H2O���ʴ�Ϊ��3NO2-+2H+=NO3-+2NO��+H2O��
��������1����ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��
��2��ʹβ����NO��NO2��ʯ�����ֽӴ�����������Ҫ�ɷ���Ca��OH��2��
��3����n��NO����n��NO2����1��1����һ����������������1��1�����������������
��4�����������غ�͵���غ㶨����д��
���������⿼��ѧ���ڡ����������Ķ���������ѧ��Ӧԭ���ڹ������̵�Ӧ�ã�������ԭ��Ӧ��������ط�Ӧ����д���ȷ����Ԫ�ػ��������ʼ���ת����ϵ�������Ӧ�ó̶ȣ�����ѧ��������Ϣ�Ĵ�����������Ŀ�Ѷ����У�
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��ʹβ����NO��NO2��ʯ�����ֽӴ���NO��NO2��������գ�������Ҫ�ɷ���Ca��OH��2���ʴ�Ϊ��ʹβ����NO��NO2��������գ�Ca��OH��2��
��3����n��NO����n��NO2����1��1����һ�������������ŷ�������NO�������ߣ���n��NO����n��NO2����1��1�����������������������������ʯ���鷴Ӧ����Ca��NO3��2���ʴ�Ϊ���ŷ�������NO�������ߣ���ƷCa��NO2��2��Ca��NO3��2�������ߣ�
��4����Ӧ����NO2-��H+����������һ���������������ˮ����Ӧ�����ӷ���ʽΪ3NO2-+2H+=NO3-+2NO��+H2O���ʴ�Ϊ��3NO2-+2H+=NO3-+2NO��+H2O��
��������1����ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��
��2��ʹβ����NO��NO2��ʯ�����ֽӴ�����������Ҫ�ɷ���Ca��OH��2��
��3����n��NO����n��NO2����1��1����һ����������������1��1�����������������
��4�����������غ�͵���غ㶨����д��
���������⿼��ѧ���ڡ����������Ķ���������ѧ��Ӧԭ���ڹ������̵�Ӧ�ã�������ԭ��Ӧ��������ط�Ӧ����д���ȷ����Ԫ�ػ��������ʼ���ת����ϵ�������Ӧ�ó̶ȣ�����ѧ��������Ϣ�Ĵ�����������Ŀ�Ѷ����У�

��ϰ��ϵ�д�
�����Ŀ


 N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��
 N2O3(g)����ƽ�ⳣ������ʽΪK=
��
N2O3(g)����ƽ�ⳣ������ʽΪK=
��
 N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��