��Ŀ����
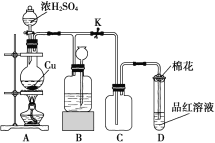
����Ŀ����ͼA��B��C��ij����С����Ƶ���ȡ������������Ȫʵ�������װ��ʾ��ͼ����ȡNH3ѡ���Լ���ͼ��ʾ���ش��������⣺
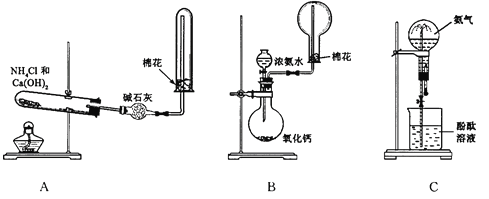
��1����Aͼ��ʾ��װ�ÿ��Ʊ������NH3
�ٷ�Ӧ�Ļ�ѧ����ʽΪ��____________________________��װ�����ռ�NH3���Թܿڷ������ŵ�������_________________________________________��
�ڸ�����и�����ܷ������ˮCaCl2___________��������_______________________��
��2����Bͼ��ʾ��װ�ÿɿ�����ȡ�ϴ���NH3������Ȫʵ�顣����Bͼ��ʾ��װ�ü��Լ��ش��������⣺
���û�ѧ����ʽ��ʾŨ��ˮ����CaO���д���NH3�ݳ��Ĺ��̣�_________________________
�ڼ���NH3�Ƿ��ռ�����ʵ�鷽���ǣ�______________________________________________��
��3����Cͼ��ʾ��װ�ý�����Ȫʵ�飬�ϲ���ƿ�ѳ������ﰱ����������Ȫ�IJ�����_______________________���÷�Ӧ��ԭ����_____________________��
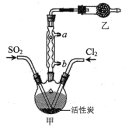
��4������ȼ����һ�㲻����Ԫ�أ���β��ȴ������NO��������ԭ���û�ѧ����ʽ��ʾ_________��������������װ�д�ת�����ɼ���β���Ի�������Ⱦ������β���е��к�����CO��NO��Ӧ��ת��Ϊ�������ŷţ�д����ط�Ӧ�Ļ�ѧ����ʽ��___________________
���𰸡�Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O ��С����������ʹ���������Թ� ���� NH3���Ժ��Ȼ��Ʒ�Ӧ CaO+H2O=Ca��OH��2����Ӧ���ȣ�NH3H2O=NH3��+H2O �ò�����պȡ����Ũ������ռ�NH3���Թܿڣ����������̣�˵���Թ����ռ���NH3����֮����û���ռ���������ʪ��ĺ�ɫʯ����ֽ�����ռ�NH3���Թܿڣ���ʪ��ĺ�ɫʯ����ֽ��������˵��NH3���ռ�������֮����û���ռ��� ��ѹ��ͷ�ιܣ���ֹˮ�� ����ˮ�ܽ����������ʹ��ƿ��ѹǿԶС��������ѹ������ˮ������ƿ N2+O2
CaCl2+2NH3��+2H2O ��С����������ʹ���������Թ� ���� NH3���Ժ��Ȼ��Ʒ�Ӧ CaO+H2O=Ca��OH��2����Ӧ���ȣ�NH3H2O=NH3��+H2O �ò�����պȡ����Ũ������ռ�NH3���Թܿڣ����������̣�˵���Թ����ռ���NH3����֮����û���ռ���������ʪ��ĺ�ɫʯ����ֽ�����ռ�NH3���Թܿڣ���ʪ��ĺ�ɫʯ����ֽ��������˵��NH3���ռ�������֮����û���ռ��� ��ѹ��ͷ�ιܣ���ֹˮ�� ����ˮ�ܽ����������ʹ��ƿ��ѹǿԶС��������ѹ������ˮ������ƿ N2+O2![]() 2NO 2NO+2CO
2NO 2NO+2CO N2+2CO2
N2+2CO2
��������
(1)��ʵ���Ҽ�����ʯ�Һ��Ȼ�炙�Ϲ�����ȡ����������ʽΪCa(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O�����������壬���Ϳ��������������������������Ƿ�ֹ����������ʹNH3�����Թܣ�
CaCl2+2NH3��+2H2O�����������壬���Ϳ��������������������������Ƿ�ֹ����������ʹNH3�����Թܣ�
���Ȼ��ƺͰ����ܷ�����ӦCaCl2+8NH3=CaCl28NH3�����Ը�����и�������ܸ�����ˮCaCl2��
(2)�������ƺ�ˮ��Ӧ�����������ƣ��ҷų����������������ƺ�ˮ�ų��������ܴٽ�һˮ�ϰ��ķֽ⣬����ʽΪ��CaO+H2O=Ca(OH)2����Ӧ���ȣ�NH3H2O![]() NH3��+H2O��
NH3��+H2O��
�ڰ�����ʹʪ��ĺ�ɫʯ����ֽ�������������Ȼ��ⷴӦ���ɰ��̣����Կ����Ȼ����ʪ��ĺ�ɫʯ����ֽ���飬�������Ϊ���ò�����պȡ����Ũ������ռ�NH3���Թܿڣ����������̣�˵���Թ����ռ���NH3����֮����û���ռ���������ʪ��ĺ�ɫʯ����ֽ�����ռ�NH3���Թܿڣ���ʪ��ĺ�ɫʯ����ֽ��������˵��NH3���ռ�������֮����û���ռ�����
(3)NH3�����ܽ���ˮ����ֹˮ�м�����ͷ�ι��е�ˮ�����ڰ���Ѹ���ܽ����ƿ������ѹǿѸ�ټ�С��������Һ������ƿ������Ȫ����
(4)�����еĵ����ڸ��������±�����������NO������ʽΪN2+O2![]() 2NO��CO��NO��Ӧ��ת��Ϊ�����壬����Ԫ���غ��֪������ӦΪN2��CO2�����ݵ����غ��֪����ʽΪ2NO+2CO
2NO��CO��NO��Ӧ��ת��Ϊ�����壬����Ԫ���غ��֪������ӦΪN2��CO2�����ݵ����غ��֪����ʽΪ2NO+2CO N2+2CO2��
N2+2CO2��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ��1994���ŵ������ѧ������Ϊ�о������������Ļ�ѧ�ҡ�O3�������к������ߣ�����������������Ŀռ䡣O3�ķ��ӽṹ��ͼ����V�ͣ�����O----O���ļн�Ϊ116.5o������ԭ����һ��Oԭ��Ϊ���ģ���������Oԭ�ӷֱ�һ�����ۼ����м�Oԭ���ṩ2�����ӣ��Ա�����Oԭ�Ӹ��ṩһ�����ӣ�����һ������Ļ�ѧ����������Oԭ�Ӿ��ȵ�����������ӡ���ش�

��1�������������Ĺ�ϵ��_____________��
��2��д�����з�����O3���ӵĽṹ�����Ƶ���_______________��
A��H2O | B��CO2 | C��SO2 | D��BeCl2 |
��3��������ijԭ����һ�Ի�û�и�����ԭ�ӹ��õļ۵��ӽй¶Ե��ӣ���ôO3������________�Թ¶Ե��ӡ�
��4��O3�����Ƿ�Ϊ���Է���____________�����ǻ��
��5��O3����ǿ�����ԣ���������PbSΪPbSO4��O2���ܣ�����ƽ��
_____PbS +___O3=________PbSO4+_______O2
����1mol O2��ת�Ƶ������ʵ���Ϊ___________mol��