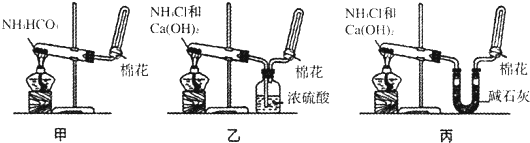
��Ŀ����
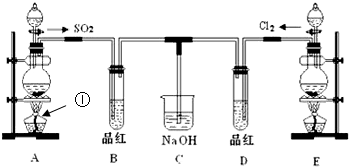
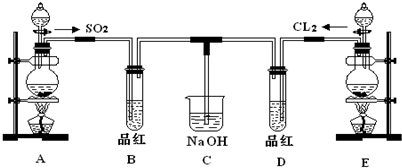
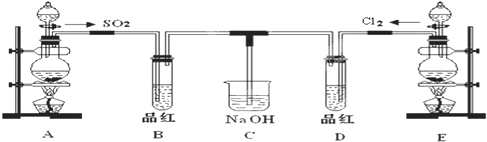
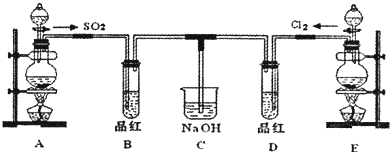
ij��ѧʵ��С���ͬѧΪ��̽���ͱȽ�SO2��Cl2ˮ��Ư���ԣ���������µ�ʵ��װ�ã�
��1�����Eװ�������Եķ�����______
��2��ʵ������װ��E�Ʊ�Cl2���壬д��E�з�����Ӧ�����ӷ���ʽ��______��
��3����Ӧ��ʼ��һ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�______������д��ţ�
�ٶ���ɫ�������ڶ�����ɫ��������B��ɫ��D����ɫ����������B����ɫ��D��ɫ
ֹͣͨ�����ٸ�B��D�����Թܼ��ȣ������Թ��е�����ֱ�ΪB��______��D��______��
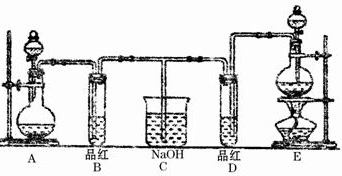
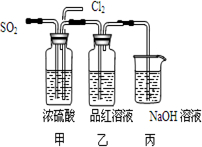
��4���ڶ���ʵ��С���ͬѧ��ΪSO2��Cl2ˮ����Ư���ԣ�����Ϻ��Ư���Ի��ǿ�����ǽ��Ƶõ�SO2��Cl2ͬʱͨ�뵽Ʒ����Һ�У������ɫЧ������������������죮Ϊ��̽��SO2��Cl2��1��1ͨ���Ư��Ч�����������������ͼʵ��װ�ã�
��ʵ�鿪ʼ����װ���г��ֵ�����______
�������ӷ���ʽ��ʾ���в����������ԭ��______
�⣺��1�����ü���Բ����ƿ�ķ������۲��Ƿ����������ɵķ��������Ƿ�©������������Ϊ��D��װˮ��û����һ�ξ��룬�رշ�Һ©���������þƾ��Ƽ�����ƿ������D��������ð����ֹͣ������Һ����������˵�����������ã�
�ʴ�Ϊ����D��װˮ��û����һ�ξ��룬�رշ�Һ©���������þƾ��Ƽ�����ƿ������D��������ð����ֹͣ������Һ����������˵�����������ã�
��2��ʵ������MnO2��Ũ�����ڼ��ȵ��������Ʊ���������Ӧ�����ӷ���ʽΪMnO2+4H++2Cl- Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4H++2Cl- Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��3�������Ͷ�����������Ʒ����Һ��ɫ�����Ƕ�������Ư������ʾ��в��ȶ��ԣ�����ʱ���ܱ�Ϊ��ɫ����������Ư���в������ԣ�
�ʴ�Ϊ���٣�����ɫ��Ϊ��ɫ������������
��4��SO2��Cl2��1��1ͨ�룬SO2��Cl2ǡ�÷�Ӧ�����߷�Ӧ����H2SO4��HCl��������SO2+Cl2+2H2O=4H++SO42-+2Cl-�����پ���Ư���ԣ�
�ʴ�Ϊ����Ʒ��δ��ɫ�� ��SO2+Cl2+2H2O=4H++SO42-+2Cl-��
��������1�����ü���Բ����ƿ�ķ������۲��Ƿ����������ɵķ��������Ƿ�©����
��2��ʵ������MnO2��Ũ�����ڼ��ȵ��������Ʊ���������Ӧ�����ӷ���ʽΪMnO2+4H++2Cl- Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��3�������Ͷ�����������Ʒ����Һ��ɫ��
��4��SO2��Cl2��1��1ͨ�룬SO2��Cl2ǡ�÷�Ӧ�����߷�Ӧ����H2SO4��HCl��������SO2+Cl2+2H2O=4H++SO42-+2Cl-�����پ���Ư���ԣ�
���������⿼�������Ͷ�����̼Ư���ԵĶԱ�ʵ�飬��Ŀ�Ѷ��еȣ�����ע����ߵ����ʵ���ͬ���ر���Ư��ԭ���IJ�ͬ�������״���Ϊ��4��������ʱע�⣮
�ʴ�Ϊ����D��װˮ��û����һ�ξ��룬�رշ�Һ©���������þƾ��Ƽ�����ƿ������D��������ð����ֹͣ������Һ����������˵�����������ã�
��2��ʵ������MnO2��Ũ�����ڼ��ȵ��������Ʊ���������Ӧ�����ӷ���ʽΪMnO2+4H++2Cl-
 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-
 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O����3�������Ͷ�����������Ʒ����Һ��ɫ�����Ƕ�������Ư������ʾ��в��ȶ��ԣ�����ʱ���ܱ�Ϊ��ɫ����������Ư���в������ԣ�
�ʴ�Ϊ���٣�����ɫ��Ϊ��ɫ������������
��4��SO2��Cl2��1��1ͨ�룬SO2��Cl2ǡ�÷�Ӧ�����߷�Ӧ����H2SO4��HCl��������SO2+Cl2+2H2O=4H++SO42-+2Cl-�����پ���Ư���ԣ�
�ʴ�Ϊ����Ʒ��δ��ɫ�� ��SO2+Cl2+2H2O=4H++SO42-+2Cl-��
��������1�����ü���Բ����ƿ�ķ������۲��Ƿ����������ɵķ��������Ƿ�©����
��2��ʵ������MnO2��Ũ�����ڼ��ȵ��������Ʊ���������Ӧ�����ӷ���ʽΪMnO2+4H++2Cl-
 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O����3�������Ͷ�����������Ʒ����Һ��ɫ��
��4��SO2��Cl2��1��1ͨ�룬SO2��Cl2ǡ�÷�Ӧ�����߷�Ӧ����H2SO4��HCl��������SO2+Cl2+2H2O=4H++SO42-+2Cl-�����پ���Ư���ԣ�
���������⿼�������Ͷ�����̼Ư���ԵĶԱ�ʵ�飬��Ŀ�Ѷ��еȣ�����ע����ߵ����ʵ���ͬ���ر���Ư��ԭ���IJ�ͬ�������״���Ϊ��4��������ʱע�⣮

��ϰ��ϵ�д�
�����Ŀ
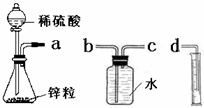 ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮��1��Ϊ�ﵽ��ʵ��Ŀ����װ������˳��Ϊ��
a��
��2�����Ӻ�װ�ú����һ��������
��3����ƿ�з�����Ӧ�����ӷ���ʽΪ
��4������װ�õķ�Һ©����װ���Լ��ֱ�Ϊ1mol/L�����4mol/L���ᣬ��С��ͬѧҪ�ⶨ����¼���������±���
| ������Լ� | H2���������ͬ�����£� | ��Ӧʱ�� | ��Ӧ���� |
| 1mol/L���� | 10mL | t1 | v1 |
| 4mol/L���� | 10mL | t2 | v2 |
��5������һ��ͬѧ�ⶨ��ÿ��һ���ӣ���ƿ�������Ũ�ȣ���¼������£�
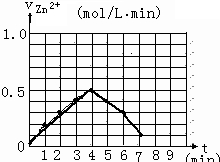
| ʱ�䣨min�� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ����Ũ�ȣ�mol/L | 4.0 | 3.8 | 3.5 | 3.1 | 2.6 | 2.2 | 1.9 | 1.8 | �� |
�����0��4mimʱ�û�ѧ��Ӧ������ʱ��仯��ԭ��
��6��������ʵ�鷽���ɶ����ⶨ�÷�Ӧ�Ļ�ѧ��Ӧ�����⣬�������е�ʵ��ⶨ�����У�