��Ŀ����
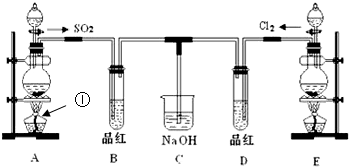
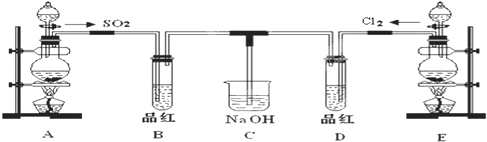
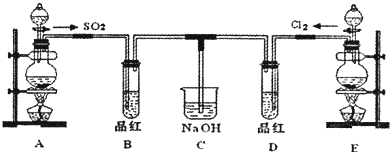
ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�ã�
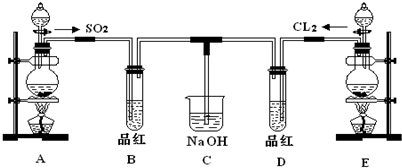
��1���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�
B��
��ֹͣͨ�����ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ��
B��
��2��ʵ����NaOH��Һ�������ǣ�

��1���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�
B��
Ʒ����ɫ
Ʒ����ɫ
��D��Ʒ����ɫ
Ʒ����ɫ
����ֹͣͨ�����ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ��
B��
����ɫ��Ϊ��ɫ
����ɫ��Ϊ��ɫ
��D������������
����������
����2��ʵ����NaOH��Һ�������ǣ�
���ն���Ķ����������������ֹ��Ⱦ����
���ն���Ķ����������������ֹ��Ⱦ����
��д��Cl2ͨ��NaOH��Һ�еĻ�ѧ����ʽCl2+2NaOH=NaCl+NaClO+H2O
Cl2+2NaOH=NaCl+NaClO+H2O
����������1�������Ͷ���������ʹƷ����Һ��ɫ��
��2�������Ͷ��������ж�������ֱ���ſգ������Ͷ��������ܺ�����������Һ��Ӧת��Ϊ�����ʣ�
�������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��2�������Ͷ��������ж�������ֱ���ſգ������Ͷ��������ܺ�����������Һ��Ӧת��Ϊ�����ʣ�
�������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ��
�����1���������Ͷ�����������Ʒ����Һ��ɫ������B��Dװ����Ʒ�춼��ɫ��
�ʴ�Ϊ��Ʒ����ɫ��Ʒ����ɫ��
�ڶ�������Ư������ʾ��в��ȶ��ԣ�����ʱ���ܱ�Ϊ��ɫ����������Ư���в������ԣ�
���Կ�����������B����Һ����ɫ��Ϊ��ɫ��D������������
�ʴ�Ϊ������ɫ��Ϊ��ɫ������������
��2�������Ͷ��������ж�������ֱ���ſգ������Ͷ��������ܺ�����������Һ��Ӧת��Ϊ�����ʣ���������������Һ�������ǣ����ն���Ķ����������������ֹ��Ⱦ�������������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��
�ʴ�Ϊ�����ն���Ķ����������������ֹ��Ⱦ������Cl2+2NaOH=NaCl+NaClO+H2O��
�ʴ�Ϊ��Ʒ����ɫ��Ʒ����ɫ��
�ڶ�������Ư������ʾ��в��ȶ��ԣ�����ʱ���ܱ�Ϊ��ɫ����������Ư���в������ԣ�
���Կ�����������B����Һ����ɫ��Ϊ��ɫ��D������������
�ʴ�Ϊ������ɫ��Ϊ��ɫ������������
��2�������Ͷ��������ж�������ֱ���ſգ������Ͷ��������ܺ�����������Һ��Ӧת��Ϊ�����ʣ���������������Һ�������ǣ����ն���Ķ����������������ֹ��Ⱦ�������������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��
�ʴ�Ϊ�����ն���Ķ����������������ֹ��Ⱦ������Cl2+2NaOH=NaCl+NaClO+H2O��
���������⿼���˶�������ʹ������Ư���ԣ��ѶȲ���ע���������ʹ�����Ư���Ե�����Ϊ�״��㣮

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
�����Ŀ
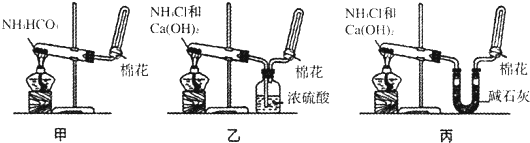
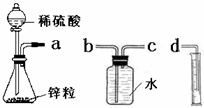 ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮
ij��ѧʵ��С���ͬѧ������������װ������ȫ��ͬ��װ�ö���̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죮��1��Ϊ�ﵽ��ʵ��Ŀ����װ������˳��Ϊ��
a��
��2�����Ӻ�װ�ú����һ��������
��3����ƿ�з�����Ӧ�����ӷ���ʽΪ
��4������װ�õķ�Һ©����װ���Լ��ֱ�Ϊ1mol/L�����4mol/L���ᣬ��С��ͬѧҪ�ⶨ����¼���������±���
| ������Լ� | H2���������ͬ�����£� | ��Ӧʱ�� | ��Ӧ���� |
| 1mol/L���� | 10mL | t1 | v1 |
| 4mol/L���� | 10mL | t2 | v2 |
��5������һ��ͬѧ�ⶨ��ÿ��һ���ӣ���ƿ�������Ũ�ȣ���¼������£�
| ʱ�䣨min�� | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ����Ũ�ȣ�mol/L | 4.0 | 3.8 | 3.5 | 3.1 | 2.6 | 2.2 | 1.9 | 1.8 | �� |
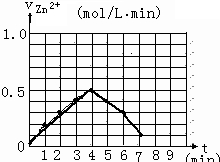
�����0��4mimʱ�û�ѧ��Ӧ������ʱ��仯��ԭ��
��6��������ʵ�鷽���ɶ����ⶨ�÷�Ӧ�Ļ�ѧ��Ӧ�����⣬�������е�ʵ��ⶨ�����У�