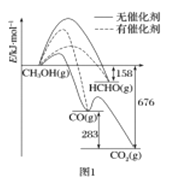
��Ŀ����
����Ŀ��ʵ��С��ģ�ҵ�Ϻ�ˮ���壬�������ʵ�顣 �ش��������⣺
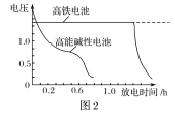
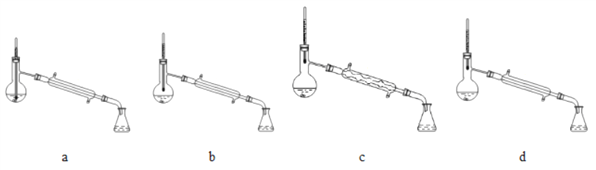
�� ������ͼ��ʾװ�ø����壺
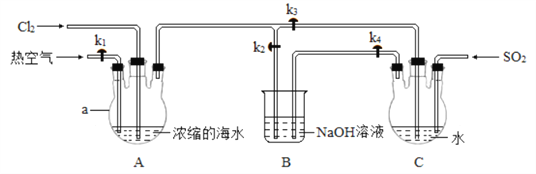
ʵ�鲽�裺
�ٹر� k1��k3����k2����װ��A ��ͨ������Cl2����ַ�Ӧ��
��ֹͣͨCl2���ر�k2���� k1��k3 ��k4�� ��װ��A ��ͨ�������ȿ�����ͬʱ��װ��C��ͨ������SO2����ַ�Ӧ��
��ֹͣͨ���壬�ر�k1��k4��
��1��a ������Ϊ___________��
��2�� ���������Ҫ��Ӧ�����ӷ���ʽΪ_____________��
��3�� �������ͨ���ȿ���������Ϊ_______�� װ�� C ����������ķ�Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ____________��ʵ�ʲμӷ�Ӧ��SO2 �����ʵ�����������ֵ����Ҫԭ��Ϊ_____________���û�ѧ����ʽ��ʾ����
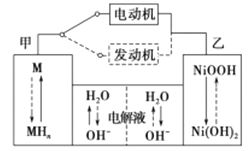
���Ʊ��壺
������Ĺ��̽���������װ�� C ��ͨ�� Cl2����ַ�Ӧ������
��4����������װ����ȷ����_____����ѡ����ĸ����
��5�� װ�� A �м���V mL �� Br����Ũ��Ϊ c mol��L��1��Ũ����ˮ�����������嵥�ʵ�����Ϊ m g�� ���ʵ�����嵥�ʵIJ���Ϊ__________��
���𰸡� ������ƿ 2Br����Cl2��Br2��2Cl�� �����ɵ� Br2����װ�� C �� 1:1 2SO2��O2��2H2O��2H2SO4 d ![]()
����������1��a������Ϊ������ƿ����2�����������Ҫ��Ӧ���������������ӣ���Ӧ�����ӷ���ʽΪ2Br����Cl2��Br2��2Cl������3�����ӷ�����˲������ͨ���ȿ���������Ϊ�����ɵ� Br2����װ��C�С�װ��C ����������ķ�Ӧ��SO2+Br2+2H2O��H2SO4+2HBr������SO2�ǻ�ԭ���������������������������뻹ԭ�������ʵ���֮��Ϊ1:1������SO2����Һ���ױ���������������ʵ�ʲμӷ�Ӧ��SO2�����ʵ�����������ֵ����Ӧ�Ļ�ѧ����ʽΪ2SO2��O2��2H2O��2H2SO4����4���������¶ȼ�ˮ���������ƿ֧�ܳ��ڴ���Ϊ��ֹҺ��������������У�Ӧ����ֱ�������ܣ���ѡd����5��װ�� A �м���V mL�� Br����Ũ��Ϊ c mol��L��1��Ũ����ˮ�����������嵥�ʵ�����Ϊm g����˸�ʵ�����嵥�ʵIJ���Ϊ![]() ��
��

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�