��Ŀ����
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���� �� �� (����)��
| A����֪2H2(g)��O2(g)===2H2O(g)����H����483��6 kJ��mol��1����������ȼ����Ϊ241��8 kJ��mol��1 |
| B����֪NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57��3 kJ��mol��1����40��0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�57��3 kJ������ |
| C����֪2C(s)��2O2(g)===2CO2(g)����H��a��2C(s)��O2(g)===2CO(g)����H��b����a>b |
| D����֪C(ʯī��s)===C(���ʯ��s)����H>0����ʯī�Ƚ��ʯ�ȶ� |
D
���������A��ȼ������ָ1mol������ȫȼ���������ȶ��Ļ��������ų���������������ȼ���ȱ�����1mol����ȼ������Һ̬ˮ�Ĺ������ų�������������B����֪NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57��3 kJ��mol��1������Ϊ������ʣ���Ӧ�����в��ϵ��룬����ĵ������ȣ���40��0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�������С��57��3 kJ������C��̼��ȫȼ�շų����������ڲ���ȫȼ�շų����������ʱ��Ǹ�ֵ����a��b������D����֪C��s��ʯī���TC��s�����ʯ����H��0��ʯī����С�ڽ��ʯ����ʯī�Ƚ��ʯ�ȶ�����ȷ��

��ϰ��ϵ�д�
�����Ŀ
 NH4++NH2����ij�¶��������ӻ�����Ϊ1��10��30��mol��L��1��2��Һ���е�pNH4��ˮ�е�pH���ƣ���1LҺ���м���2.3gNa��ʱ����ƽ��____________�ƶ���������������淽����������ȫ��Ӧ����Һ��pNH4=__________��
NH4++NH2����ij�¶��������ӻ�����Ϊ1��10��30��mol��L��1��2��Һ���е�pNH4��ˮ�е�pH���ƣ���1LҺ���м���2.3gNa��ʱ����ƽ��____________�ƶ���������������淽����������ȫ��Ӧ����Һ��pNH4=__________��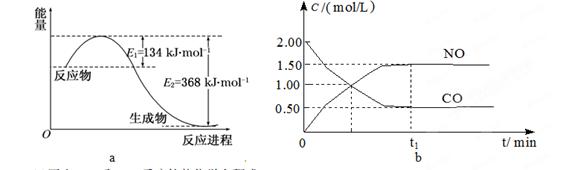

 CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)�����ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
 ��ԭΪN2��һ��ʱ�����Һ�ļ���
��ԭΪN2��һ��ʱ�����Һ�ļ���