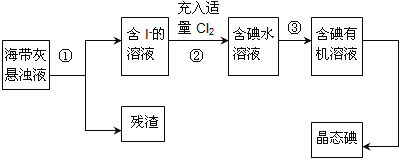
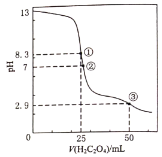
��Ŀ����
����Ŀ���������֪ʶ��գ�
(1)��������SO2��SO3������ԭ�Ӹ���֮��__________
(2)4.8gCH4��������ԭ�Ӹ�����____________gˮ������ԭ�������
(3)12.4gNa2R��Na+0.4mol����Na2R��Ħ������Ϊ_____________��R�����ԭ��������_________
(4)�����, 16g������̼��һ����̼��ɵĻ������,�����Ϊ8.96L,��û��������ܶ��������Ϊ________��һ����̼�Ͷ�����̼�����ʵ���֮��Ϊ___________,�����˻������ͨ�������ij���ʯ��ˮ�У����ɵij�����������___________��
���𰸡�5��6 10.8 62g/mol 16 20 1��3 30g
��������
(1)��������ʵ���֮�ȣ���ͬ���ʵ�����SO2��SO3����Oԭ�ӵ����ʵ���֮��Ϊ2��3���ݴ˷�����
(2)4.8g������Hԭ�ӵ����ʵ���Ϊ![]() =1.2mol��Ȼ��ͨ��ˮ��Hԭ�����ʵ���Ϊ1.2mol�������H2O��������
=1.2mol��Ȼ��ͨ��ˮ��Hԭ�����ʵ���Ϊ1.2mol�������H2O��������
(3)Na2R��Na��de���ʵ���Wie0.4mol�������Na2R�����ʵ���������n=![]() �����Na2R��Ħ���������Ӷ������R��Ħ��������
�����Na2R��Ħ���������Ӷ������R��Ħ��������
(4)������ͬ�����£��ܶ�֮�ȵ���Ħ������֮�ȣ������������ƽ��Ħ���������ݴ˷�����
(1)��ͬ������SO2��SO3�����ʵ���֮��Ϊ![]() ��
��![]() =5��4�������ͬ������SO2��SO3����ԭ�����ʵ���֮��Ϊ(5��2)��(4��3)=5��6��
=5��4�������ͬ������SO2��SO3����ԭ�����ʵ���֮��Ϊ(5��2)��(4��3)=5��6��
�𰸣�5��6��
(2) 4.8g������Hԭ�ӵ����ʵ���Ϊ![]() =1.2mol�������
=1.2mol�������![]() =1.2mol�����m(H2O)=10.8g��
=1.2mol�����m(H2O)=10.8g��
�𰸣�10.8��
(3)1molNa2R�к���2molNa��������0.4molNa����Na2R�����ʵ���Ϊ0.2mol������n=![]() ��0.2mol=
��0.2mol=![]() ���ó�M=62g/mol��R��Ħ������Ϊ(62��2��23)g��mol��1=16g��mol��1����R�����ԭ������Ϊ16��
���ó�M=62g/mol��R��Ħ������Ϊ(62��2��23)g��mol��1=16g��mol��1����R�����ԭ������Ϊ16��
�𰸣�62g��mol��1��16��
(4)�����������ʵ���Ϊ![]() =0.4mol�����������ƽ��Ħ������Ϊ
=0.4mol�����������ƽ��Ħ������Ϊ![]() =40g��mol��1����ͬ�����£�������ܶ�֮�ȵ�����Ħ������֮�ȣ������������ܶ��������Ϊ
=40g��mol��1����ͬ�����£�������ܶ�֮�ȵ�����Ħ������֮�ȣ������������ܶ��������Ϊ![]() =20������������n(CO)��n(CO2)=0.4mol��28n(CO)��44n(CO2)=16g���������n(CO)=0.1mol��n(CO2)=0.3mol����CO��CO2�����ʵ���֮��Ϊ0.1��0.3=1��3��ֻ��CO2����Ca(OH)2��Ӧ������CO2��Ca(OH)2=CaCO3����H2O�������n(CO2)=n(CaCO3)=0.3mol������������Ϊ0.3mol��100g��mol��1=30g��
=20������������n(CO)��n(CO2)=0.4mol��28n(CO)��44n(CO2)=16g���������n(CO)=0.1mol��n(CO2)=0.3mol����CO��CO2�����ʵ���֮��Ϊ0.1��0.3=1��3��ֻ��CO2����Ca(OH)2��Ӧ������CO2��Ca(OH)2=CaCO3����H2O�������n(CO2)=n(CaCO3)=0.3mol������������Ϊ0.3mol��100g��mol��1=30g��
�𰸣�20��1��3��30g��

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�����Ŀ��ij���жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5��ֱ��С�ڵ���2.5��m�����������������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش���������
��1����PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
Ũ��/mol��L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
���ݱ��������жϴ�������Ϊ__����ᡱ����ԣ���ʾ����������Ե�c(H+)��c(OH-)=___mol��L-1��
��2��úȼ���ŷŵ������к���SO2��NOx�����γ����꣬��Ⱦ����������NaClO2��Һ�ڼ��������¿ɶ�������������������Ч���dz��á�������ж������������̵����ӷ���ʽ��
��____��ClO2-+��____��NO+��____��OH-=��____��Cl-+��____��NO3-+______
��3��Ϊ����SO2�Ի�������Ⱦ������ú̿ת��Ϊ��������ȼ�ϣ������������д������������е�SO2��
��д����̿��ˮ������Ӧ�Ļ�ѧ����ʽ��__��
���������ʿ�����������������SO2����__������ĸ���ţ���
a.Ca(OH)2 b.Na2CO3 c.CaCl2 d.NaHSO3
��4������β����NOx��CO�����ɼ�ת����
����������ʱ�����¶ȸߣ������л�����NO����ѧ����ʽΪ___��
������ȼ�Ͳ���ȫȼ��ʱ����CO��������β��ϵͳ��װ�ϴ�ת������ʹCO��NO��Ӧת��Ϊ����Ⱦ�����Ե��������壬�仯ѧ��Ӧ����ʽΪ___��