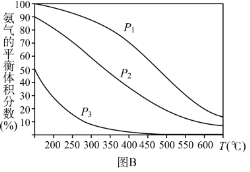
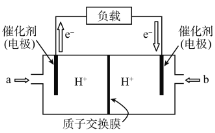
��Ŀ����
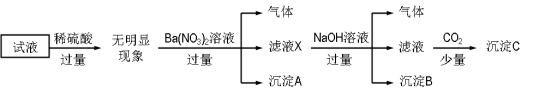
����Ŀ���ڵ�Ƴ���ĺ������Է�ˮ�У����Ĵ�����ʽ��Cr(��)��Cr(��)���֣�������Cr(��)�Ķ������ⷨ����������ˮ��ͼ����������Cr(OH)3������ȥ������˵����ȷ����
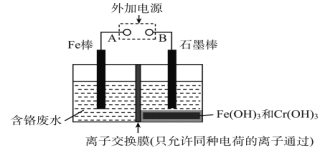
A. FeΪ��������ӦΪFe��2e��=Fe2+
B. ������ӦΪCr2O72��+7H2O+6e��=2Cr(OH)3��+8OH��
C. ����ÿת��3mol���ӣ��ɴ���Cr(��)���ʵ���Ϊ1mol
D. ���ӽ���ĤΪ���ӽ���Ĥ��ֻ����H+����
���𰸡�A
��������
��ⷨ����������ˮ��ԭ���������������������ڵ���������������������������Ӧ�����������ӣ������������£��������ӽ����۸����ӻ�ԭ�����۸����ӣ��������ӱ�������������������ͬʱ����������ˮ�ŵ�������������OH-��OH-����Һ��Cr3+��Fe3+��Ӧ�γ�������������������ﵽ��ˮ������Ŀ�ġ�
A��ڵ���������������������������Ӧ�����������ӣ��缫��ӦʽΪFe��2e��=Fe2+����A��ȷ��
B�Cr2O72������������Fe2+��ԭΪCr3+��������ˮ�ŵ�ŵ�������������OH-���缫��ӦʽΪ2H2O+2e��= H2��+2 OH������B����
C�����ÿת��3mol���ӣ���1.5mol Fe2+���ɣ������ӷ���ʽCr2O72-+6Fe2++14H+=2Cr3+
+7H2O+6Fe3+��֪��1.5mol Fe2+��ԭ0.25molCr2O72-�����ɴ���Cr(��)���ʵ���Ϊ0.5mol����C����
D�Cr2O72������������Fe2+��Ӧ����Cr3+��Fe3+��������ˮ�ŵ�������������OH-��Cr3+��Fe3+ͨ�������ӽ���Ĥ��������������OH-��Ӧ����������������������ӽ���Ĥ�������ӽ���Ĥ���������ӽ���Ĥ����D����
��ѡA��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���й�ʮ�Ŵ�ָ�����ӿ�ˮ��Ⱦ���Ρ�ʵʩ�����ͽ��������ۺ�������������Ⱦ�������ǻ�ѧ�������о�����Ҫ���⣬Ҳ�Ǽ��С���ˮ��ɽ���ǽ�ɽ��ɽ������Ҫ�ٴ롣���ʵ��������£���CO2ת��Ϊ�״������ѵ��л���ȿɽ���CO2��ɵ�����ЧӦ�Ի�����Ӱ�죬���ɵõ���Ҫ���л����
(1)��֪����2H2(g)+O2(g)=2H2O(g) ��H1=484 kJ/mol
��CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H2=50 kJ/mol
CH3OH(g)+H2O(g) ��H2=50 kJ/mol
��2CH3OH(g)+3O2(g)= 2CO2(g)+4H2O(g) ��H����H=_____________��
(2)��֪T Kʱ��ij�����ܱ������д������·�Ӧ��2CO2(g)+6H2(g) ![]() CH3OCH3(g)+3H2O(g) ��H<0����������в�ͬʱ��ʱ�����ʵ�Ũ��(mol/L)���±���ʾ��
CH3OCH3(g)+3H2O(g) ��H<0����������в�ͬʱ��ʱ�����ʵ�Ũ��(mol/L)���±���ʾ��
c(CO2) | c(H2) | c(CH3OCH3) | c(H2O) | |
��ʼʱ | a | b | 0 | 0 |
10 sʱ | 3 | 0.5 | c | 1.5 |
������Ӧ��ʼ��10sʱ����ڣ�v(H2)=_____________��������߷�Ӧ���ʣ��������H2ת���ʵķ�����________________________��
����T Kʱ����ѧƽ�ⳣ��K=15����10 s ʱv(��)_______v(��)���>����<����=��������ʱCO2��ת����Ϊ________��
(3)һ�������£���ij�����ܱ������г���x mol CO2��y mol H2�������ķ�ӦΪCO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H=50 kJ��mol1��
CH3OH(g)+H2O(g) ��H=50 kJ��mol1��
����ͼ1���ܱ�ʾ�÷�Ӧ��ƽ�ⳣ��K���¶�T֮��ı仯��ϵ����Ϊ______���a����b���������ж�������__________________________________��
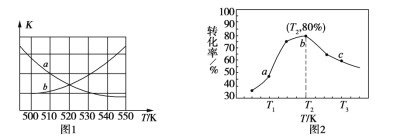
����x=2��y=3���������ͬʱ���ڲ�ͬ�¶���H2��ת������ͼ2��ʾ�����ڸ�ʱ����ڣ�ǡ�ôﵽ��ѧƽ��ʱ�������ڵ�ѹǿ�뷴Ӧ��ʼʱ��ѹǿ֮��Ϊ____________��