��Ŀ����
��Ϊ�˳�ȥKCl��Һ��������Mg2����SO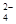 ����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
��1�������Լ��У� B�� ��C�� ��
(2)������������� ��
(3)�ӹ���Aʱ�����йط�Ӧ�����ӷ���ʽΪ �� ��
��һ����ɫϡ��Һ�п��ܺ���Na+��Fe3+��H+��Mg2����CO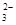 ��OH����HCO
��OH����HCO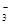 ��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
����ʯ����ֽ����Һ������ԣ���ֽ����ɫ��
��ȡ2������Һ���ȼ�����ϡ�����ữ��������������ټ��Ȼ�����Һ���м��飬û����������
��1��ԭ��Һ��һ�����ڵ������� ��һ�������ڵ������� ��
��2�������������������ܿ϶��Ƿ���ڵ������� ���������һ����ʵ�鷽�����ж��Ƿ�����������ӡ�
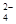 ����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²�������� 
��1�������Լ��У� B�� ��C�� ��
(2)������������� ��
(3)�ӹ���Aʱ�����йط�Ӧ�����ӷ���ʽΪ �� ��
��һ����ɫϡ��Һ�п��ܺ���Na+��Fe3+��H+��Mg2����CO
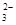 ��OH����HCO
��OH����HCO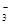 ��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²���������ʯ����ֽ����Һ������ԣ���ֽ����ɫ��
��ȡ2������Һ���ȼ�����ϡ�����ữ��������������ټ��Ȼ�����Һ���м��飬û����������
��1��ԭ��Һ��һ�����ڵ������� ��һ�������ڵ������� ��
��2�������������������ܿ϶��Ƿ���ڵ������� ���������һ����ʵ�鷽�����ж��Ƿ�����������ӡ�
��(1)K2CO3��1�֣��� HCl��1�֣��� (2)�����ᾧ��1�֣���
��3��Mg2++2OH��=Mg(OH)2����2�֣�;Ba2++SO42��=BaSO4����2�֣���
��1��Na+,CO32��,OH����2�֣���Fe3+,H+,Mg2+,HCO3����2�֣�����ȫ�Ե�2�֣�©д��1�֣��д����÷֣�
(2)Cl����1�֣��� ȡ����ԭ��Һ����������ϡ�����ữ���ٵμ�����AgNO3,���а�ɫ��������������Cl��,����û�С���2�֣�
��3��Mg2++2OH��=Mg(OH)2����2�֣�;Ba2++SO42��=BaSO4����2�֣���
��1��Na+,CO32��,OH����2�֣���Fe3+,H+,Mg2+,HCO3����2�֣�����ȫ�Ե�2�֣�©д��1�֣��д����÷֣�
(2)Cl����1�֣��� ȡ����ԭ��Һ����������ϡ�����ữ���ٵμ�����AgNO3,���а�ɫ��������������Cl��,����û�С���2�֣�
���������
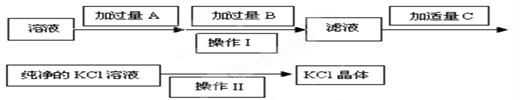
��(1�������������Խ�MgCl2��MgSO4�е����������������ȥ������ʱ��ÿһ�����ӵ��Լ����ǹ����ģ�������������������̼��س�ȥ���������̼���������������ȥ�����Լ�A��B��C������Ba��OH��2��K2CO3��HCl��
��2��Һ��������ķ����ǹ��ˣ����ò���������©�������������ձ������ʵ���Һ��þ���ķ����������ᾧ��
��3�������������Խ�MgCl2��MgSO4�е����������������ȥ������ʽ�ֱ�Ϊ��MgCl2+Ba��OH��2�TBaCl2+Mg��OH��2����MgSO4+Ba��OH��2�TBaSO4��+Mg��OH��2����
��1����ʯ����ֽ����Һ������ԣ���ֽ����ɫ,˵����Һ�ʼ��ԡ���OH-���ӷ�Ӧ�����Ӳ��ܹ��棬Fe3+��H+��Mg2����HCO
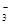 �ų���������ϡ�����ữ�������������˵������CO
�ų���������ϡ�����ữ�������������˵������CO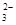 ������ԭ��Һ��һ�����ڵ�������Na+����Һ�����ٺ���һ�������ӣ�,CO32��,OH����һ�������ڵ�������Fe3+,H+,Mg2+,HCO3���������ܿ϶��Ƿ���ڵ�������Cl����ȡ����ԭ��Һ����������ϡ�����ữ���ٵμ�����AgNO3,���а�ɫ��������������Cl��,����û�С�
������ԭ��Һ��һ�����ڵ�������Na+����Һ�����ٺ���һ�������ӣ�,CO32��,OH����һ�������ڵ�������Fe3+,H+,Mg2+,HCO3���������ܿ϶��Ƿ���ڵ�������Cl����ȡ����ԭ��Һ����������ϡ�����ữ���ٵμ�����AgNO3,���а�ɫ��������������Cl��,����û�С�
��ϰ��ϵ�д�
�����Ŀ
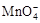 ����C2O42?�����ӷ���ʽ����������
����C2O42?�����ӷ���ʽ����������
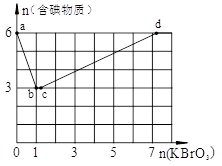
 Na++H++SO42һ
Na++H++SO42һ