��Ŀ����
ȡ����������Һ�����������е����ӽ��м��飬�����ж���ȷ����
| A���������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ���壬��������һ����CO32�� |
| B������KSCN��Һ�������������ٵμӼ���������ˮ����Һ��ΪѪ��ɫ����������һ����Fe2+ |
| C������BaCl2��Һ��������ɫ�������ټ��������ϡ�����ữ���������ܽ⣬��������һ����SO42�� |
| D���ھƾ��ƻ��������գ�����Ϊ��ɫ����������һ������Ԫ�أ�һ��������Ԫ�� |
B
���������A�����ܺ���HCO3��������C�����ܺ���Ag��������D�����ܺ��м�Ԫ�أ�����

��ϰ��ϵ�д�
�����Ŀ
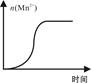

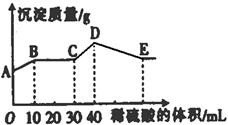


 ����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²�������� 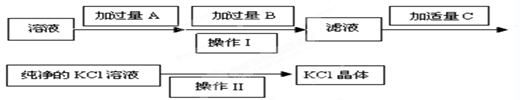
 ��OH����HCO
��OH����HCO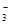 ��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����