��Ŀ����
����A��B��C��D��E��F���ֳ����������֪���ǰ�������������K����Ag����Na����
Ba2����Fe2����Al3������������Cl����OH����AlO2-��NO3-��SO42-��CO32-�������Ƿֱ����0.1 mol/L����Һ��������ʵ�飺
�ٲ����ҺA��C��E���ʼ��ԣ��Ҽ���A��E��C��E����ɫ��dz��ɫ������ɫ�ܲ����۲죩������B��Һ�еμ�ϡ��ˮ�������������ɳ����������ȫ���ܽ⣻����F��Һ�еμ�ϡ���ᣬ��Һ����ػ�ɫ��������ɫ�������ɣ�����D��Һ�еμ�Ba��NO3��2��Һ����������
��1��д��A��D��E��F�Ļ�ѧʽ��
A________��D________��E________��F________��
��2�������ӷ���ʽ����C��Һ�ʼ��Ե�ԭ��__________________________________��
��3��д��ʵ����з�Ӧ�����ӷ���ʽ��________________________________��
Ba2����Fe2����Al3������������Cl����OH����AlO2-��NO3-��SO42-��CO32-�������Ƿֱ����0.1 mol/L����Һ��������ʵ�飺
�ٲ����ҺA��C��E���ʼ��ԣ��Ҽ���A��E��C��E����ɫ��dz��ɫ������ɫ�ܲ����۲죩������B��Һ�еμ�ϡ��ˮ�������������ɳ����������ȫ���ܽ⣻����F��Һ�еμ�ϡ���ᣬ��Һ����ػ�ɫ��������ɫ�������ɣ�����D��Һ�еμ�Ba��NO3��2��Һ����������
��1��д��A��D��E��F�Ļ�ѧʽ��
A________��D________��E________��F________��
��2�������ӷ���ʽ����C��Һ�ʼ��Ե�ԭ��__________________________________��
��3��д��ʵ����з�Ӧ�����ӷ���ʽ��________________________________��
��1��Ba��OH��2 AlCl3 KAlO2 FeSO4
��2��CO32-��H2O HCO3-��OH��
HCO3-��OH��
��3��3Fe2����NO3-��4H��=3Fe3����NO����2H2O
��2��CO32-��H2O
 HCO3-��OH��
HCO3-��OH����3��3Fe2����NO3-��4H��=3Fe3����NO����2H2O
ʹ��Һ�ʼ��Ե���������OH����AlO2-��CO32-������ʵ��ڿ���ȷ��BΪAgNO3������ʵ��ۿ���ȷ��F����ΪFeCl2����FeSO4������ʵ��ܿ���ȷ��D�����ܺ���SO42-������FΪFeSO4��DΪAlCl3��������������������С��̼������ģ�����ƫ�����εļ���ǿ��̼���εģ���ϼ���A��E��C������ȷ��AΪBa��OH��2��EΪKAlO2��CΪNa2CO3��C��Һ�ʼ��Ե�ԭ��CO32-��H2O HCO3-��OH����ʵ����з�Ӧ�����ӷ���ʽΪ3Fe2����NO3-��4H��=3Fe3����NO����2H2O��
HCO3-��OH����ʵ����з�Ӧ�����ӷ���ʽΪ3Fe2����NO3-��4H��=3Fe3����NO����2H2O��
 HCO3-��OH����ʵ����з�Ӧ�����ӷ���ʽΪ3Fe2����NO3-��4H��=3Fe3����NO����2H2O��
HCO3-��OH����ʵ����з�Ӧ�����ӷ���ʽΪ3Fe2����NO3-��4H��=3Fe3����NO����2H2O��
��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ
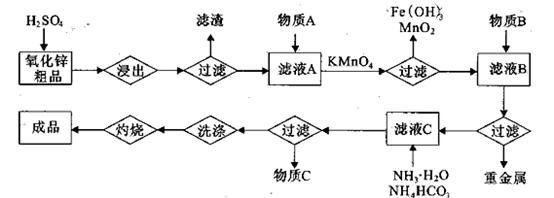
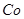 ����Mn2����
����Mn2����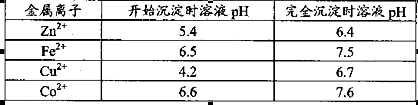
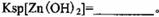 ��
��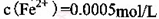 ������1
������1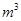 ����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���
����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���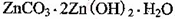 �����ɸó����Ļ�ѧ����ʽΪ________��
�����ɸó����Ļ�ѧ����ʽΪ________��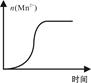

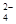 ����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²��������
����ѡ��Ba(OH)2��HCl��K2CO3�����Լ��������²�������� 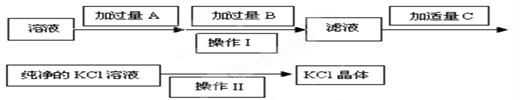
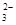 ��OH����HCO
��OH����HCO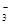 ��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����
��Cl���������е������֡�Ϊ��ȷ����Һ����ɣ����������²�����