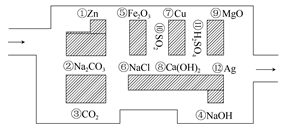
��Ŀ����
�й�ʮ�˴�������������ƽ���̬�������衱��
�� ȼú�����Ӵ���PM2.5��ֵ���γ������������������֮һ��ú�������Ǹ�Ч����������ú̿����Ҫ;����д�����ȵĽ�̿��ˮ������Ӧ�Ļ�ѧ����ʽ ��
�� ����ҵ�����ġ��ع��͡�����Ҫ�ɷ�����֬���ۺ����á��ع��͡���һ�ַ����ǽ����ع��͡��е���֬ˮ���Ի�ȡ��֬����� �������ƣ������������Ͻ��� ���������Ի����ϩ����ϩ�Ȼ���ԭ�ϡ�
�� �����ؽ�����Ⱦ��2013��ȫ�������������ص㡣����Hg2+�ķ�ˮ�м���Na2S������ʹHg2+ת��ɳ��������ӷ�Ӧ����ʽΪ ��
�� �ҹ���������һ��ɷ�Ϊ�����Ĵ��ࣺ�ɻ��������������������к����������������������������ڿɻ����������� ������ĸ����
a. �ϱ�ֽ b. ������������ c. ����ҩƷ d. ����
�� C + H2O 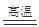 CO + H2
CO + H2
�ڸ��� �ѽ⣨�����ѽ� ��
�� Hg2+ + S2��= HgS��
�� a b
��������������ٽ�̿��ˮ������Ӧ�ɵ�ˮú��CO��H2����ѧ����ʽΪC + H2O  CO + H2 ��
CO + H2 ��
����֬�ijɷ��Ǹ�֬����ĸ�������ˮ��������֬������ͣ�������ϩ���ľۺ�����������Ͻ����ѽ���ɵ���ϩ����ϩ�ȣ�
������Hg2+�ķ�ˮ�м���Na2S������HgS�ij��������ӷ���ʽΪHg2+ + S2��= HgS����
�ܷϱ�ֽ���������������ǿɻ��������������������ã�����ҩƷ���ɻ��գ����뵹�����������ռ�ֵ����ѡab��
���㣺������ˮú���ķ�Ӧ����֬ˮ�������жϣ����ӷ���ʽ����д�������������ж�

 ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д���������������Һ���ܴ����������
| A��Fe3+��SCN����I����K+ |
| B��K+��Al3+��SO42����MnO4�� |
| C��H+��NO3����Fe2+��Na+ |
| D��Cu2+��NH4+��Br����OH�� |
 K2S��N2����3CO2��
K2S��N2����3CO2��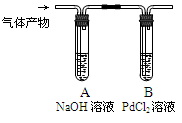
 ��
�� ��X�е�һ�֡�
��X�е�һ�֡�