��Ŀ����
17�������£��������һԪ��MOH��Һ�������ϣ�������Һ����仯����������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±��������жϴ�����ǣ�������| ʵ���� | �����Ũ�ȣ�mol?L-1�� | MOH��Һ��Ũ�ȣ�mol?L-1�� | �����Һ��pH |
| �� | 0.2 | 0.2 | a |
| �� | 0.2 | c1 | 7 |
| �� | 0.1 | 0.1 | 5 |
| A�� | ��������Һ��M+ˮ��̶Ƚϱ����д���a��5 | |
| B�� | ��������Һ�У�c��Cl-����c��M+����c��H+����c��OH-�� | |
| C�� | ��������Һ�У�c��M+��+c��MOH����0.1mol?L-1����c1��0.2 | |
| D�� | ��������Һ�У�c��OH-��+c��MOH��=1��10-5 mol?L-1 |
���� ���ݱ��е�Ũ�ȵ��Ȼ�����MOH��Ӧǡ������MCl�����ڻ��Һ��pH=5����˵��MOHΪ���
A�����������������������ӵ�Ũ��Խ��ˮ��̶�ԽС��������Һ�е����������ӣ��������ӣ�Ũ��Խ��
B��������Һ������ΪMCl��M+���Ӳ���ˮ�⣬c��Cl-����c��M+������Һ��ʾ���ԣ�c��H+����c��OH-����
C����c1=0.2ʱ����Ӧ����MCl����Һ��ʾ���ԣ���Ҫ��Һ��ʾ���ԣ���MOH��Ũ��Ӧ���Դ�
D������MCl��Һ�еĵ���غ�������غ�ɵã�c��OH-��+c��MOH��=c��H+������Һ��pH=5����c��H+��=10-5mol/L��
��� �⣺����ʵ���У����������Ũ�ȵ�HCl��MOHǡ�÷�Ӧ����MCl��������Һ��pH=5��֪��MClΪǿ�������Σ�MOHΪ���
A���ͱ��Ļ��Һ�����ʶ���MCl�����м�Ũ�ȴ��ڱ�����������Һ��M+ˮ��̶�С�ڱ��飻��Һ��������Ũ�ȣ��ף���������Һ��pH���ף���������a��5����A����
B�����鷴Ӧ��Ļ��Һ������ΪMCl������M+���Ӳ���ˮ�⣬M+����Ũ�ȼ�С����c��Cl-����c��M+����M+����ˮ����Һ��ʾ���ԣ���c��H+����c��OH-������Һ������Ũ�ȴ�СΪ����������Һ�У�c��Cl-����c��M+����c��H+����c��OH-������B��ȷ��
C�����c1=0.2����Ũ�ȡ��������HCl��MOHǡ�÷�Ӧ����MCl����Һ��ʾ���ԣ�����ҺpH=7����MOH�����ʵ���Ũ��Ӧ�ô���HCl����c1��0.2�����������غ��֪��c��M+��+c��MOH����c��Cl-��=0.1mol/L����C��ȷ��
D������ΪMCl����Һ�����ݵ���غ�ɵã���c��Cl-��+c��OH-��=c��M+��+c��H+�������������غ�ɵã���c��M+��+c��MOH��=c��Cl-�������ڴ��ˢٿɵã�c��OH-��+c��MOH��=c��H+��=10-5mol/L����D��ȷ��
��ѡA��
���� ���⿼����������ʵĵ��롢�ε�ˮ��ԭ��������Ũ�ȴ�С�Ƚϵ�֪ʶ����Ŀ�Ѷ��еȣ�ע�������ε�ˮ��ԭ����������ʵĵ��뼰��Ӱ�죬�ܹ����ݵ���غ㡢�����غ㡢�ε�ˮ���ж���Һ������Ũ�ȴ�С����������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �Ȼ�����Һ�е�����������Һ | B�� | ̼��������ȼ�� | ||
| C�� | ��Ƭ��������ͭ��Һ�� | D�� | ����������Һ��ϡ���ᷴӦ |
| A�� | ��ʪ���pH��ֽ��ϡ��Һ��pH���ⶨֵƫС | |
| B�� | �ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС | |
| C�� | ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС | |
| D�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ֵƫС |
| A�� | ��״���£�1mol Cl2��HCl�Ļ������������������Ϊ2NA | |
| B�� | 101kPa��0��ʱ��22.4 L H2����ԭ����ΪNA�� | |
| C�� | ��״���£�2.24 L CCl4���е�̼ԭ����ĿΪ0.1NA | |
| D�� | ���³�ѹ��3.2 g O2�к��е���ԭ����ĿΪ0.2 NA |
| A�� | -44.2 kJ/mol | B�� | +44.2 kJ/mol | C�� | -330 kJ/mol | D�� | +330 kJ/mol |
| A�� | ��������ƽ��ȡ3.23 g NaCl���� | |
| B�� | ��10 mL ��Ͳ��ȡ 7.50 mL ϡ���� | |
| C�� | ��Һ�Ժ� �²�Һ��ӷ�Һ©���¶˹ܿڷų����رջ�������һ�������������ϲ�Һ������ӷ�Һ©���¶˹ܿڷų� | |
| D�� | ϡ��Ũ����ʱ����Ũ��������������ע��ˮ������Ͻ��� |
| A�� | �������������ƾ��������Һ��pH������ | |
| B�� | ���¶�������50�棬����Һ��pH������ | |
| C�� | ��ˮϡ����ԭ�����2��������Һ��pH����С | |
| D�� | ��������п�۳�ַ�Ӧ������Һ��������һ���� |
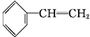 +Br2��
+Br2��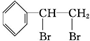 ����Ӧ�����Ǽӳɷ�Ӧ��
����Ӧ�����Ǽӳɷ�Ӧ�� ��
��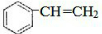 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$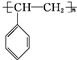 ��
��