��Ŀ����
�����ڹ�ũҵ����������ҪӦ�á�
��1���ٵ������ڹ�ҵ�ϳɰ���д�������ĵ���ʽ ��
��NH3���ȶ��Ա�PH3 ����д��ǿ������������
��2������ͼ��ʾ����NaOH�����ϵμ���Ũ��ˮ��Ѹ�ٸ��ϸǣ��۲�����
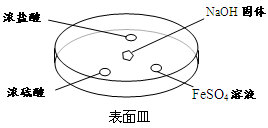
��Ũ����Һ�θ�������ְ��̣�������Ӧ�Ļ�ѧ����ʽΪ ��
��Ũ����Һ���Ϸ�û����������һ��ʱ���Ũ�����Һ�����а�ɫ���壬�ù�������� ��д��ѧʽ��һ�ּ��ɣ���
��FeSO4Һ�����ȳ��ֻ���ɫ��������һ��ʱ����ɺ��ɫ�������ķ�Ӧ����
Fe2++2NH3��H2O=Fe(OH)2��+ 2NH4+ �� ��
��3���������ѷ���Ŀǰ����NH3��ˮ����Ⱦ����Ҫ��������һ�������£���ˮ���м�������NaOH��ʹNH3���ѳ���������ƽ���ƶ�ԭ��������ԭ�� ��
��4�������������£���������ˮ�зֽ�����İ��ܹ��������������������ᣨHNO2������Ӧ�Ļ�ѧ����ʽΪ ������Ӧ����0.3 mol���ӷ���ת��ʱ�����������������Ϊ g��С���������λ��Ч���֣���
��1���ٵ������ڹ�ҵ�ϳɰ���д�������ĵ���ʽ ��
��NH3���ȶ��Ա�PH3 ����д��ǿ������������
��2������ͼ��ʾ����NaOH�����ϵμ���Ũ��ˮ��Ѹ�ٸ��ϸǣ��۲�����

��Ũ����Һ�θ�������ְ��̣�������Ӧ�Ļ�ѧ����ʽΪ ��
��Ũ����Һ���Ϸ�û����������һ��ʱ���Ũ�����Һ�����а�ɫ���壬�ù�������� ��д��ѧʽ��һ�ּ��ɣ���
��FeSO4Һ�����ȳ��ֻ���ɫ��������һ��ʱ����ɺ��ɫ�������ķ�Ӧ����
Fe2++2NH3��H2O=Fe(OH)2��+ 2NH4+ �� ��
��3���������ѷ���Ŀǰ����NH3��ˮ����Ⱦ����Ҫ��������һ�������£���ˮ���м�������NaOH��ʹNH3���ѳ���������ƽ���ƶ�ԭ��������ԭ�� ��
��4�������������£���������ˮ�зֽ�����İ��ܹ��������������������ᣨHNO2������Ӧ�Ļ�ѧ����ʽΪ ������Ӧ����0.3 mol���ӷ���ת��ʱ�����������������Ϊ g��С���������λ��Ч���֣���
��1����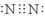 ��1�֣� ��ǿ��1�֣�
��1�֣� ��ǿ��1�֣�
��2����NH3+HCl=NH4Cl��1�֣� ��NH4HSO4��(NH4)2SO4��1�֣�
��4Fe(OH)2+O2+2H2O=4Fe(OH)3��2�֣�
��3������ˮ�д���ƽ�⣺NH3+H2O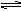 NH3��H2O
NH3��H2O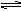 NH4+ +OH-��1�֣�д��NH3+H2O
NH4+ +OH-��1�֣�д��NH3+H2O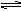 NH4+ +OH-���۷֣�������NaOH��OH-Ũ������ƽ�������ƶ���1�֣����������ڰ����ѳ���2�֣�
NH4+ +OH-���۷֣�������NaOH��OH-Ũ������ƽ�������ƶ���1�֣����������ڰ����ѳ���2�֣�
��4��2NH3+3O2 =2HNO2+2H2O��2�֣���д���ﲻ�۷֣� 2.35 g��2�֣�
=2HNO2+2H2O��2�֣���д���ﲻ�۷֣� 2.35 g��2�֣�
 ��1�֣� ��ǿ��1�֣�
��1�֣� ��ǿ��1�֣���2����NH3+HCl=NH4Cl��1�֣� ��NH4HSO4��(NH4)2SO4��1�֣�
��4Fe(OH)2+O2+2H2O=4Fe(OH)3��2�֣�
��3������ˮ�д���ƽ�⣺NH3+H2O
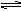 NH3��H2O
NH3��H2O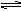 NH4+ +OH-��1�֣�д��NH3+H2O
NH4+ +OH-��1�֣�д��NH3+H2O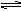 NH4+ +OH-���۷֣�������NaOH��OH-Ũ������ƽ�������ƶ���1�֣����������ڰ����ѳ���2�֣�
NH4+ +OH-���۷֣�������NaOH��OH-Ũ������ƽ�������ƶ���1�֣����������ڰ����ѳ���2�֣���4��2NH3+3O2
 =2HNO2+2H2O��2�֣���д���ﲻ�۷֣� 2.35 g��2�֣�
=2HNO2+2H2O��2�֣���д���ﲻ�۷֣� 2.35 g��2�֣������������1���ٵ���������Nԭ�Ӽ��γ�3�Թ��õ��ӣ�������Ϊ��
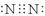
��NԪ�طǽ�����ǿ��PԪ�أ�����NH3���ȶ��Ա�PH3ǿ��
��2����Ũ����ӷ�����HCl��Ũ��ˮ�ӷ�����NH3��Ӧ�����ɵİ���ΪNH4Cl����ѧ����ʽΪ��NH3+HCl=NH4Cl��
��Ũ��ˮ�ӷ�����NH3������Ũ���������ᷴӦ���ɣ�NH4HSO4��(NH4)2SO4��
�����ձ�Ϊ���ɫ��������Fe(OH)3����ѧ����ʽΪ��4Fe(OH)2+O2+2H2O=4Fe(OH)3��
��3��NH3?H2OΪ������ڵ���ƽ�⣺NH3+H2O
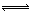 NH3?H2O
NH3?H2O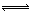 NH4++OH?������NaOH��������OH?Ũ�ȣ�ʹƽ�����淴Ӧ�����ƶ��������ڰ����ѳ���
NH4++OH?������NaOH��������OH?Ũ�ȣ�ʹƽ�����淴Ӧ�����ƶ��������ڰ����ѳ�����4��NH3��O2��Ӧ�����������ˮ����ƽ�ɵû�ѧ����ʽ��2NH3+3O2
 2HNO2+2H2O�����ݻ��ϼ۵ı仯��֪��6��n��HNO2��=0.3mol��n��HNO2��=0.05mol����m��HNO2��=2.35g��
2HNO2+2H2O�����ݻ��ϼ۵ı仯��֪��6��n��HNO2��=0.3mol��n��HNO2��=0.05mol����m��HNO2��=2.35g��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
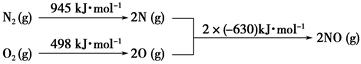
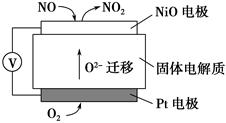
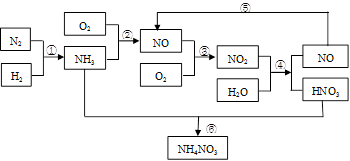
 ____NO+ ________
____NO+ ________ 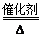 7N2��12H2O(NOҲ�����Ƶķ�Ӧ)
7N2��12H2O(NOҲ�����Ƶķ�Ӧ)