��Ŀ����
��1��д�����з�Ӧ�����ӷ��̣�
����NaOH��Һͨ������������̼��
����NaHSO4��Һ����μ���Ba��OH��2��Һ�����ԣ�
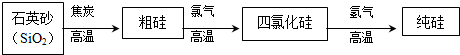
��2�����̿�MnO2�������Ĺ���KOH��KClO3�ڸ����·�Ӧ����������أ�K2MnO4����KCl��д�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ��
����NaOH��Һͨ������������̼��
CO2+2OH-�TCO32-+H2O
CO2+2OH-�TCO32-+H2O
������NaHSO4��Һ����μ���Ba��OH��2��Һ�����ԣ�
Ba2++SO42-+2OH-+2H+=BaSO4��+2H2O
Ba2++SO42-+2OH-+2H+=BaSO4��+2H2O
����2�����̿�MnO2�������Ĺ���KOH��KClO3�ڸ����·�Ӧ����������أ�K2MnO4����KCl��д�����̿��Ʊ�K2MnO4�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ��
3MnO2+6KOH+KClO3
3K2MnO4+KCl+3H2O��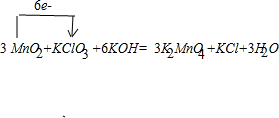
| ||
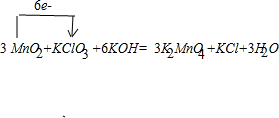
3MnO2+6KOH+KClO3
3K2MnO4+KCl+3H2O��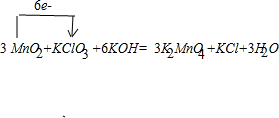
��
| ||
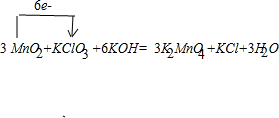
��������1���ٸ��ݶ�����̼���������������������̼��Ӧ����̼����д�����ӷ���ʽ��
�ڵ������Ӻ�����������ǡ����ȫ��Ӧʱ��ҺΪ���ԣ�
��2�����ݷ�Ӧ��������̡��������ء������������������ء��Ȼ�����д��Ӧ����ʽ�����ݻ��ϼ۱仯�������ת�Ƶķ������Ŀ��
�ڵ������Ӻ�����������ǡ����ȫ��Ӧʱ��ҺΪ���ԣ�
��2�����ݷ�Ӧ��������̡��������ء������������������ء��Ȼ�����д��Ӧ����ʽ�����ݻ��ϼ۱仯�������ת�Ƶķ������Ŀ��
����⣺��1������������������������̼��Ӧ�����ӷ���ʽΪ��CO2+2OH-�TCO32-+H2O��
�ʴ�Ϊ��CO2+2OH-�TCO32-+H2O��
����NaHSO4��Һ�е���Ba��OH��2��Һ����������ǡ�ñ���ȫ�к�ʱ��ҺΪ���ԣ��������ӷ���ʽΪBa2++SO42-+2OH-+2H+=BaSO4��+2H2O��
�ʴ�Ϊ��Ba2++SO42-+2OH-+2H+=BaSO4��+2H2O��
��2�������̿����������KOH��KClO3�ڸ����·�Ӧ����������أ�K2MnO4����KCl����ӦΪ3MnO2+6KOH+KClO3
3K2MnO4+KCl+3H2O������ת�Ƶķ������ĿΪ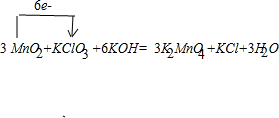 ��
��
�ʴ�Ϊ��3MnO2+6KOH+KClO3
3K2MnO4+KCl+3H2O��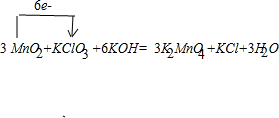 ��
��
�ʴ�Ϊ��CO2+2OH-�TCO32-+H2O��
����NaHSO4��Һ�е���Ba��OH��2��Һ����������ǡ�ñ���ȫ�к�ʱ��ҺΪ���ԣ��������ӷ���ʽΪBa2++SO42-+2OH-+2H+=BaSO4��+2H2O��
�ʴ�Ϊ��Ba2++SO42-+2OH-+2H+=BaSO4��+2H2O��
��2�������̿����������KOH��KClO3�ڸ����·�Ӧ����������أ�K2MnO4����KCl����ӦΪ3MnO2+6KOH+KClO3
| ||
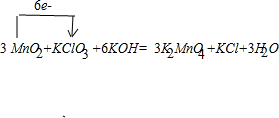 ��
���ʴ�Ϊ��3MnO2+6KOH+KClO3
| ||
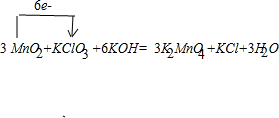 ��
�����������⿼��ѧ�����ӷ���ʽ����д֪ʶ�����Ը�����ѧ�������ش������ڿ��Ե��ȵ㣬�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ