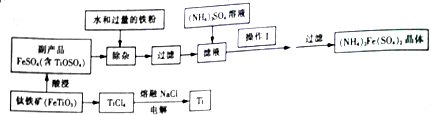
��Ŀ����
11��ij��ѧ��ȤС������������Ҫ�ɷ�ΪAl2O3��������SiO2�������������ȡ��������ұ������ԭ�ϣ���ȡ�IJ���������ͼ1��ʾ��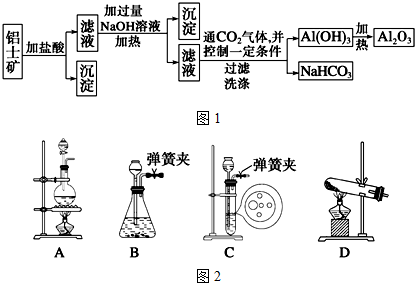
��1��ʵ��������ȡCO2ʱ��Ϊ��ʹ��Ӧ�濪���á������ͣ��Ӧѡ����ͼ2�е�װ��C������ĸ���ţ���
��2���ڹ��˲����У����ձ����������⣬�����õ��IJ���������©����ϴ�ӳ����IJ�������©�����������ˮ��ʹˮ���û��������ˮ��Ȼ������ظ�2��3�Σ�
��3��ʵ�����Ʊ����������ķ����ж��֣����ṩ��м������������Һ��ϡ��������ҩƷ�����Ʊ����������������������ҩƷ�������ٵĽǶȳ�������Ƴ����ʵ�鷽�����������ظ�������д���˷����з�����Ӧ�����ӷ���ʽ2Al+6H+�T2Al3++3H2����2Al+2OH-+2H2O�T2AlO2-+3H2����Al3++3AlO2-+6H2O�T4Al��OH��3�������˷���������ҩƷ�����ʵ���֮��n��Al����n��H2SO4����n��NaOH��=8��3��6��
��4����ȤС����������������Ԫ�صļ�̬����̽����ȡ�������壬�������ϡ���ᣬ�����ܽ⣻ȡ������Һ�μ�KSCN��Һ����ֺ�ɫ���ɴ˵ó�����Ԫ�صļ�̬Ϊ+3�Ľ��ۣ���ָ���ý����Ƿ������˵�����ɲ�������ϡ������ǿ�����ԣ������ļ�̬Ϊ+2�ۣ��ɱ�����Ϊ+3�ۣ�ͬ����KSCN��Ӧ��Һ�Ժ�ɫ����
���� �����̿�֪�������ᣬֻ��SiO2����Ӧ�����������������������ᷴӦ�����˳���ΪSiO2����Һ�мӹ���NaOH��������ת��Ϊƫ�����ƣ�������ת�����������˺���Һ���������̼��Ӧ��������������̼�����ƣ�������������ֽ�������������
��1����CO2ʱΪ��ʹ��Ӧ�濪���ã������ͣ����ҪӦ�����շ�������װ��ԭ��������ֹˮ�п�������ѹǿ��ʹҺ��������ֹͣ��Ӧ����ѹǿ��СҺ�����Ӵ�������Ӧ��
��2�����ݹ��˲�����װ��ͼ�����ж����貣���������ڹ���װ����ϴ�ӳ���������ˮ��û����ʹˮ��Ȼ���£��ظ����Σ�
��3��������Ƶ�ʵ�鷽������Ϸ�Ӧ������ϵ������
�������У�2Al+6H+=2Al3++3H2����Al3++3NH3•H2O=Al��OH��3��+3NH4+��
3 1 1 3 1
������1molAl��OH��3ʱ������3molH+��3molOH-��
��������2Al+2NaOH+2H2O=2NaAlO2+3H2����AlO2-+H2O+H+=Al��OH��3����
1 1 1 1 1 1
������1molAl��OH��3ʱ������1molH+��1molOH-��
��������2Al+6H+=2Al3++3H2����2Al+2NaOH+2H2O=2NaAlO2+3H2����Al3++3AlO2-+6H2O=4Al��OH��3����
������1molAl��OH��3ʱ������$\frac{3}{4}$molH+��$\frac{3}{4}$molOH-��
��4���������������������������������Ϊ�����ӷ�����
��� �⣺��1�����շ���������ʱ���У���ʱֹͣ��װ�ã������ڹ����Һ�岻���ȷ�Ӧ���ɲ�����ˮ�������Ʊ���Ӧ��������CO2ʱΪ��ʹ��Ӧ�濪���ã������ͣӦ�������շ�������ԭ��Ӧ�ã�Ӧ��ѡ��װ��C������ֹˮ�п�������ѹǿ���رյ��ɼк�Ӧ���ɵ�����ʹ�ϲ�ѹǿ������һ���̶Ⱥ�ɰѷ�ӦҺѹ��©����ʹҺ��������ֹͣ��Ӧ����ѹǿ��СҺ�����Ӵ�������Ӧ��
�ʴ�Ϊ��C��
��2�����ݹ��˲�����װ��ͼ�����ж����貣���������ڹ��˲����У����ձ����������⣬�����õ��IJ���������©�����ڹ���װ����ϴ�ӳ���������ˮ��û����ʹˮ��Ȼ���£�ϴ�ӳ����IJ����ǣ���©�����������ˮ��ʹˮ���û��������ˮ��Ȼ������ظ�2��3�Σ�
�ʴ�Ϊ��©������©�����������ˮ��ʹˮ���û��������ˮ��Ȼ������ظ�2��3�Σ�
��3��������1molAl��OH��3ʱ���ɷ�Ӧ����ʽ��֪
�������У�2Al+6H+=2Al3++3H2����Al3++3NH3•H2O=Al��OH��3��+3NH4+��
3 1 1 3 1
������1molAl��OH��3ʱ������3molH+��3molOH-��
��������2Al+2NaOH+2H2O=2NaAlO2+3H2����AlO2-+H2O+H+=Al��OH��3����
1 1 1 1 1 1
������1molAl��OH��3ʱ������1molH+��1molOH-��
��������2Al+6H+=2Al3++3H2����2Al+2NaOH+2H2O=2NaAlO2+3H2����Al3++3AlO2-+6H2O=4Al��OH��3����
������1molAl��OH��3ʱ������$\frac{3}{4}$molH+��$\frac{3}{4}$molOH-��
��Ȼ��������ã�ҩƷ�����٣�������Ϊ���˷���������ҩƷ�����ʵ���֮��n��Al����n��H2SO4����n��NaOH��=1��$\frac{3}{4}$��$\frac{1}{2}$��$\frac{3}{4}$=8��3��6��
�ʴ�Ϊ��2Al+6H+�T2Al3++3H2����2Al+2OH-+2H2O�T2AlO2-+3H2����Al3++3AlO2-+6H2O�T4Al��OH��3����8��3��6��
��4��ȡ�������壬�������ϡ���ᣬ�������ǿ�����ԣ������ܽ⣬ȡ������Һ�μ�KSCN��Һ����ֺ�ɫ�������ļ�̬Ϊ+2�ۣ��ɱ�����Ϊ+3�ۣ�ͬ����KSCN��Ӧ��Һ�Ժ�ɫ��
�ʴ�Ϊ����������ϡ������ǿ�����ԣ������ļ�̬Ϊ+2�ۣ��ɱ�����Ϊ+3�ۣ�ͬ����KSCN��Ӧ��Һ�Ժ�ɫ��
���� ���⿼����������ᴿ�������ۺ�Ӧ�ã�Ϊ��Ƶ���㣬���������еķ�Ӧ����ѧ��Ӧ����ʽ��дΪ���Ĺؼ������ط�����ʵ����������ѧ����Ŀ��飬��Ŀ�Ѷ��еȣ�

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�| A�� | �� | B�� | � | C�� | �� | D�� | þ |
��ij��ҵ��ˮ����Ҫ����Cr3+��ͬʱ������������Fe3+��Al3+��Ca2+��Mg2+�ȣ������Խ�ǿ��Ϊ�������ã�ͨ�������������̴�����
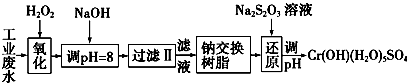
ע�����������ӳ�����������������ʽ��ȫ����ʱ��Һ��pH���±���
| �������� | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 | Al��OH��3 | Cr��OH��3 |
| pH | 3.7 | 9.6 | 11.1 | 8 | 9����9�ܽ⣩ |
A��Na2O2 B��HNO3 C��FeCl3 D��KMnO4
��2������NaOH��Һ������ҺpH=8ʱ����ȥ��������AB����֪�����ӽ�����֬��ԭ����Mn++nNaR-��MRn+nNa+���˲�������������ȥ������������CD��
A��Fe3+ B��Al3+ C��Ca2+ D��Mg2+
��3����ԭ�����У�ÿ����0.8mol Cr2O72-ת��4.8mol e-���÷�Ӧ���ӷ���ʽΪ3S2O32-+4Cr2O72-+26H+�T6SO42-+8Cr3++13H2O��
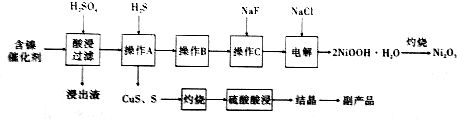
�����������£����۸���Ҫ��Cr2O72-��ʽ���ڣ���ҵ�ϳ��õ�ⷨ������Cr2O72-�ķ�ˮ��
�÷���Fe���缫��⺬Cr2O72-�����Է�ˮ�����ŵ����У�������������ҺpH���ߣ�����Cr��OH��3��Һ��
��1�����ʱ�ܷ���Cu�缫������Fe�缫�����ܣ���ܡ����ܡ�����������������������Cu2+����ʹCr2O72-��ԭ���ͼ�̬��
��2�����ʱ����������Һ��Cr2O72-ת��ΪCr3+�����ӷ���ʽΪCr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O��
��3�������£�Cr��OH��3���ܶȻ�Ksp=1��10-32����Һ��pHӦΪ5ʱ����ʹc��Cr3+������10-5 mol•L-1��
| A�� | �����淴Ӧ���ʶ������� | B�� | SO2��O2��SO3��Ũ����� | ||
| C�� | SO2��O2��SO3�������й��� | D�� | SO2��O2��SO3��Ũ�Ⱦ����ٱ仯 |
 ��
��