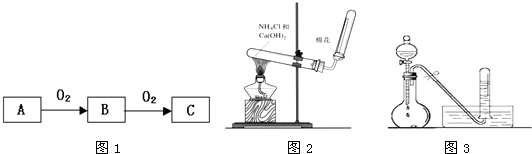
��Ŀ����
A��B��C����ѧ��ѧ�������������ʣ�����֮����ת����ϵ��ͼ1��ʾ�����ַ�Ӧ������������ȥ����
��1����A��һ�ֻ�ɫ���ʹ��壬��B��C�Ļ�ѧ����ʽΪ______��
��2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��C������Ϊ______�����û�ѧ����ʽ��ʾ�������������̼����ķ�Ӧ______����C����¶���ڿ����У���������D��D�Ļ�ѧʽΪ______��
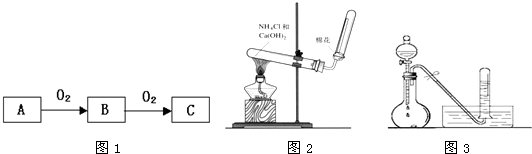
��3����C�Ǻ���ɫ���壬A������һ����ʹʪ��ĺ�ɫʯ����ֽ���������壮��ͼ2��ʾ��ʵ������ȡA�����װ�ã�������ѧ֪ʶ���ش��������⣺
���ռ�A�ķ�����______����֤A�Ƿ��Ѿ��ռ����ķ�����______����дһ�֣���
��д��ʵ������ȡA�Ļ�ѧ����ʽ______��
������5.35g�Ȼ�鱗μӷ�Ӧ���������A�����ڱ�״���µ����Ϊ______L��
����д��C��ˮ��Ӧ�Ļ�ѧ����ʽ______����Ӧ�ɵõ���X��X��______����ʣ��ǿ��������������ͼ3��ʾ������X��Ũ��Һ��Cu��Ӧ��д����ƿ�з�����Ӧ�����ӷ���ʽ______��ʵ����Ϻ��Թ����ռ������������Ҫ�ɷ�Ϊ______��д��ѧʽ��
�⣺��1����A��һ�ֻ�ɫ���ʹ��壬��A��S���ʣ�����B�Ƕ�������C������������B��C�Ļ�ѧ����ʽΪ2SO2+O2 2SO3���ʴ�Ϊ��2SO2+O2
2SO3���ʴ�Ϊ��2SO2+O2 2SO3��
2SO3��
��2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��A���ƣ�B�������ƣ�C�ǹ������ƣ���������������CO2������������Ӧ�Ļ�ѧ����ʽ��2Na2O2+2 CO2=2 Na2CO3+O2����
�ʴ�Ϊ���������ƣ� 2Na2O2+2 CO2=2 Na2CO3+O2����Na2CO3��
��3����AΪ��ʹʪ��ĺ�ɫʯ����ֽ���������壬��AӦ���ǰ�����C�Ǻ���ɫ���壬ӦΪNO2��
��AΪ��������������ˮ���ܶȱȿ���С�����������ſ������ռ������鰱���Ƿ��ռ������ɽ�ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�������ֽ��������֤���������ռ��������ð�ɫ�ķ�̪��ֽ�����Թܿڴ�������ֽ��죬��֤���������ռ�����Ҳ������պ��Ũ����IJ����������Թܿڴ����������������̣���֤���������ռ�����
�ʴ�Ϊ�������ſ���������ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�������ֽ��������֤���������ռ�����
�����ð�ɫ�ķ�̪��ֽ�����Թܿڴ�������ֽ��죬��֤���������ռ�����
������պ��Ũ����IJ����������Թܿڴ����������������̣���֤���������ռ�����
��ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ����������Ӧ�ķ���ʽΪCa��OH��2+2NH4Cl CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��n��NH4Cl��=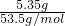 =0.1mol����n��NH3��=0.1mol��V��NH3��=2.24L��
=0.1mol����n��NH3��=0.1mol��V��NH3��=2.24L��
�ʴ�Ϊ��2.24��
��CΪNO2����ˮ��Ӧ���������NO����Ӧ�ķ���ʽΪ3NO2+H2O=2HNO3+NO��HNO3��ǿ����ʣ�����ǿ�����ԣ�����ͭ��Ӧ����Ӧ�����ӷ���ʽΪCu+4H++2NO3-=Cu2++2NO2��+2H2O��ʵ����Ϻ��Թ����ռ������������Ҫ�ɷ�ΪNO��
�ʴ�Ϊ��3NO2+H2O=2HNO3+NO��ǿ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��NO��
��������1����A��һ�ֻ�ɫ���ʹ��壬��A��S���ʣ�����B�Ƕ�������C����������
��2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��A���ƣ�B�������ƣ�C�ǹ������ƣ�
��3����AΪ��ʹʪ��ĺ�ɫʯ����ֽ���������壬��AӦ���ǰ�����C�Ǻ���ɫ���壬ӦΪNO2��
���������⿼������ͼ����жϣ��Ǹ߿��еij������ͣ����ڻ���������Ŀ��飮�����������У�����֪ʶ�����ض�ѧ������֪ʶ�Ĺ��̸�ѵ��������������Ҫע����ǻ�ѧ�ƶ�����һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ����������ѧ�Ƽ��ۺϣ��������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά���������ͼ��ķ�������ؼ�����Ѱ�ҡ�ͻ�ƿڡ�����ͻ�ƿڡ�����ץ���ء��֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȣ�
 2SO3���ʴ�Ϊ��2SO2+O2
2SO3���ʴ�Ϊ��2SO2+O2 2SO3��
2SO3����2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��A���ƣ�B�������ƣ�C�ǹ������ƣ���������������CO2������������Ӧ�Ļ�ѧ����ʽ��2Na2O2+2 CO2=2 Na2CO3+O2����
�ʴ�Ϊ���������ƣ� 2Na2O2+2 CO2=2 Na2CO3+O2����Na2CO3��
��3����AΪ��ʹʪ��ĺ�ɫʯ����ֽ���������壬��AӦ���ǰ�����C�Ǻ���ɫ���壬ӦΪNO2��
��AΪ��������������ˮ���ܶȱȿ���С�����������ſ������ռ������鰱���Ƿ��ռ������ɽ�ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�������ֽ��������֤���������ռ��������ð�ɫ�ķ�̪��ֽ�����Թܿڴ�������ֽ��죬��֤���������ռ�����Ҳ������պ��Ũ����IJ����������Թܿڴ����������������̣���֤���������ռ�����
�ʴ�Ϊ�������ſ���������ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�������ֽ��������֤���������ռ�����
�����ð�ɫ�ķ�̪��ֽ�����Թܿڴ�������ֽ��죬��֤���������ռ�����
������պ��Ũ����IJ����������Թܿڴ����������������̣���֤���������ռ�����
��ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ����������Ӧ�ķ���ʽΪCa��OH��2+2NH4Cl
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O���ʴ�Ϊ��Ca��OH��2+2NH4Cl
 CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O����n��NH4Cl��=
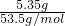 =0.1mol����n��NH3��=0.1mol��V��NH3��=2.24L��
=0.1mol����n��NH3��=0.1mol��V��NH3��=2.24L���ʴ�Ϊ��2.24��
��CΪNO2����ˮ��Ӧ���������NO����Ӧ�ķ���ʽΪ3NO2+H2O=2HNO3+NO��HNO3��ǿ����ʣ�����ǿ�����ԣ�����ͭ��Ӧ����Ӧ�����ӷ���ʽΪCu+4H++2NO3-=Cu2++2NO2��+2H2O��ʵ����Ϻ��Թ����ռ������������Ҫ�ɷ�ΪNO��
�ʴ�Ϊ��3NO2+H2O=2HNO3+NO��ǿ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��NO��
��������1����A��һ�ֻ�ɫ���ʹ��壬��A��S���ʣ�����B�Ƕ�������C����������
��2����A��һ�ֻ��ý�����C�ǵ���ɫ���壬��A���ƣ�B�������ƣ�C�ǹ������ƣ�
��3����AΪ��ʹʪ��ĺ�ɫʯ����ֽ���������壬��AӦ���ǰ�����C�Ǻ���ɫ���壬ӦΪNO2��
���������⿼������ͼ����жϣ��Ǹ߿��еij������ͣ����ڻ���������Ŀ��飮�����������У�����֪ʶ�����ض�ѧ������֪ʶ�Ĺ��̸�ѵ��������������Ҫע����ǻ�ѧ�ƶ�����һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ����������ѧ�Ƽ��ۺϣ��������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά���������ͼ��ķ�������ؼ�����Ѱ�ҡ�ͻ�ƿڡ�����ͻ�ƿڡ�����ץ���ء��֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȣ�

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ



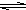 HS-+OH-
HS-+OH-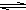 Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״�������� ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��