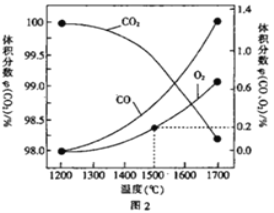
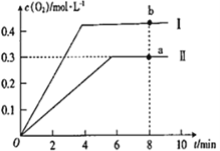
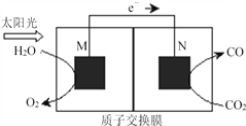
��Ŀ����
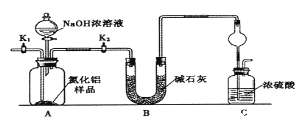
����Ŀ��ijǿ������ҺX�п��ܺ���Fe2����Fe3����Al3����Ba2����NH4+��CO32-��NO3-��SO42-��SiO32-�е������֣���ȡX��Һ��������ʵ�飬ʵ����̼�������ͼ��ʾ��ʵ���������һ������Ϊ����ɫ��

����������Ϣ���ش��������⣺
(1)��ǿ�������������ж�X��Һ�п϶������ڵ�������________________��
(2)��ҺX�й�����������ӵ��жϣ���ȷ����______(���ţ���ͬ)��
a��һ������ ��b��һ�������� ������c�����ܺ���
(3)����F�ĵ���ʽΪ____________��������I�к��еĻ�ѧ��������________________��
(4)ת���ٵ����ӷ���ʽΪ_______________________________________________________��
ת���ߵ����ӷ���ʽΪ_______________________________________________________��
(5)�Բ���ȷ���Ƿ���ڵ����ӣ�������ȡX��Һ������������Һ�е�һ�֣����������жϣ����Լ������________��
��NaOH��Һ����KSCN��Һ������ˮ��KSCN�Ļ����Һ����pH��ֽ����KMnO4��Һ
���𰸡�CO32-��SiO32-b![]() ���ۼ������Ӽ�3Fe2+ +4H++NO3-=3Fe3+ +NO��+2H2OAlO2-��2H2O��CO2=HCO3-��Al(OH)3����
���ۼ������Ӽ�3Fe2+ +4H++NO3-=3Fe3+ +NO��+2H2OAlO2-��2H2O��CO2=HCO3-��Al(OH)3����
��������
(1)��ǿ������Һ�������Ӻ�̼������ӷ�Ӧ���ɶ�����̼��ˮ����������Ӻ������ӷ�Ӧ���ɹ������������һ���������CO32-��SiO32-��
(2)����������ᱵ���ɳ����������Ӻ���������ӷ�Ӧ�������ᱵ������˵��һ������SO42-������SO42-��һ������Ba2��������A������������D��E����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2������������������һ������NO![]() ����������������NO3-�ὫFe2�����������ܴ������棬��ѡb��
����������������NO3-�ὫFe2�����������ܴ������棬��ѡb��
(3)��ҺB�м������NaOH��Һ�����ȣ���������F����FΪNH3�������ʽΪ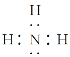 ����ҺEΪHNO3������FΪNH3��E��F��Ӧ����IΪNH4NO3�����еĻ�ѧ�����������Ӽ��ͼ��Թ��ۼ���
����ҺEΪHNO3������FΪNH3��E��F��Ӧ����IΪNH4NO3�����еĻ�ѧ�����������Ӽ��ͼ��Թ��ۼ���
(4)���������ṩ�����ӣ�ǰ�����ų�SiO32-�Ĵ��ڣ����ڹ����������������µõ�����ҺH��ͨ�����������̼���ܲ�������K��KӦ��Ϊ��������������ҺH�к���ƫ��������ӣ�����ҺX�к���Al3����ת���ߵ����ӷ���ʽΪAlO2-��2H2O�� CO2===HCO3-��Al(OH)3����
(5)���Ͽ�֪����Һ��һ������Fe2����Al3����NH4+��SO42-��һ��������Ba2����CO32-��NO3-��SiO32-������ȷ���Ƿ���Fe3�������ѡ��KSCN��Һ��Fe3�����м��飬��ѡ����

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�