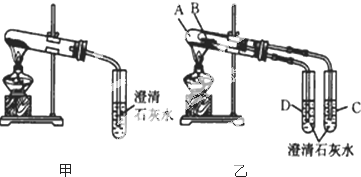
��Ŀ����
1�� ���������һ�������ǻ���������������������������Ŀǰ����Ҫ��һ��������ͼ�DZ���ͳ�Ƶ�������������Դ�ͳɷַ��������ж�����������������ʩ��������ȷ���ǣ�������
���������һ�������ǻ���������������������������Ŀǰ����Ҫ��һ��������ͼ�DZ���ͳ�Ƶ�������������Դ�ͳɷַ��������ж�����������������ʩ��������ȷ���ǣ�������| A�� | ��ֵ�ϴ��ȼú����ȥȼú����Ļҳ� | |
| B�� | �ӿ��ҹ��ɡ�ȼú��ʱ�����뵽��������ʱ���IJ��� | |
| C�� | ���ô�ת������������β���е�NO2��COת��Ϊ������ | |
| D�� | ����NO2��һ�ַ��������ü������ԭNO2����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-512kJ•mol-1����1g���鴦��NO2��Ҫ�ų�16kJ������ |
���� �����������ֺ�������������й�����������Ⱦ��ĺ����йأ����Գ�ֵ�ϴ��ȼú����ȥȼú����Ļҳ����ɼ��ٿ����й����������ŷţ��ɡ�ȼú��ʱ�����뵽��������ʱ�����ô�ת������������β���е�NO2��COת��Ϊ�����壬�ɼ��ٷ�����SO2��NO2��CO���к����ʵ��ŷţ��ݴ˷������
��� �⣺A����ֵ�ϴ��ȼú����ȥȼú����Ļҳ��������˹���۳����ŷţ�����Ч����������������A��ѡ��
B���ӿ��ҹ��ɡ�ȼú��ʱ�����뵽��������ʱ�����ɼ��ٷ�����SO2���к����ʵ��������ٻ�����Ⱦ����ŷţ�����Ч����������������B��ѡ��
C�����ô�ת������������β���е�NO2��COת��Ϊ�����壬��������Ⱦ����ŷţ�����Ч����������������C��ѡ��
D������NO2��һ�ַ��������ü������ԭNO2����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����û�м�������Ⱦ����ŷţ�������Ч����������������Dѡ��
��ѡD��
���� ���⿼�黷����Ⱦ��������Ϊ��Ƶ���㣬����PM2.5�������Ŀ��飬��Ϥ��Դ�������������ij���֮��Ĺ�ϵ���ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
11��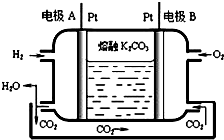 ������ѧ��ѧԭ��������������⣺
������ѧ��ѧԭ��������������⣺
��1���軯�����������ˮ���о綾������HCN���ӷ������ᣬ��֪��Ka��HCN��=6.17x10-10��������CN-��ˮʱ����NaOH��Һ������pH=9ʱ�����£���c��CN-����c��HCN�������������������=������
��2����֪��
��C��s��+O2��g���TCO2��g������H=a kJ•mol-1��
��CO2��g��+C��s���T2CO��g������H=b kJ•mol-1��
��Si��s��+O2��g���TSiO2��s������H=c kJ•mol-1��
��ҵ�������ֹ���Ȼ�ѧ����ʽΪ2C��s��+SiO2��s���TSi��s��+2CO��g����H=��a+b-c��kJ•mol-1��
��3����֪��CO��g��+H2O��g��?H2��g��+CO2��g�����±�Ϊ�÷�Ӧ�ڲ�ͬ�¶�ʱ��ƽ�ⳣ����
�÷�Ӧ�ġ�H��0�������������������500��ʱ���и÷�Ӧ����CO��H2O��ʼŨ����ȣ�COƽ��ת����Ϊ75%��
��7��һ����������ȼ�ϵ�ع���ԭ����ͼ��ʾ��д���缫A�ĵ缫��ӦʽH2-2e-+CO32-�TCO2+H2O��
 ������ѧ��ѧԭ��������������⣺
������ѧ��ѧԭ��������������⣺��1���軯�����������ˮ���о綾������HCN���ӷ������ᣬ��֪��Ka��HCN��=6.17x10-10��������CN-��ˮʱ����NaOH��Һ������pH=9ʱ�����£���c��CN-����c��HCN�������������������=������
��2����֪��
��C��s��+O2��g���TCO2��g������H=a kJ•mol-1��
��CO2��g��+C��s���T2CO��g������H=b kJ•mol-1��
��Si��s��+O2��g���TSiO2��s������H=c kJ•mol-1��
��ҵ�������ֹ���Ȼ�ѧ����ʽΪ2C��s��+SiO2��s���TSi��s��+2CO��g����H=��a+b-c��kJ•mol-1��
��3����֪��CO��g��+H2O��g��?H2��g��+CO2��g�����±�Ϊ�÷�Ӧ�ڲ�ͬ�¶�ʱ��ƽ�ⳣ����
| �¶�/ | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
��7��һ����������ȼ�ϵ�ع���ԭ����ͼ��ʾ��д���缫A�ĵ缫��ӦʽH2-2e-+CO32-�TCO2+H2O��
12����ѧ�����ǻ�ѧѧ�������������ԣ������йػ�ѧ����ʹ�ò���ȷ���ǣ�������
| A�� | �Ƶ�Ԫ�ط��ţ�Ca | B�� | �����ӽṹʾ��ͼ��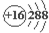 | ||
| C�� | ������̼�Ľṹʽ O=C=O | D�� | NaCl�ĵ���ʽ��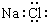 |
9�����н����У����ս����Դ�����ǿ��˳�����е��ǣ�������
| A�� | ����þ���ơ��� | B�� | þ�������ء��� | C�� | �ơ��ء�﨡��� | D�� | �ơ��ء��ơ�� |
6��������Һ����ʯ��ˮ����H2S��Һ����KMnO4��Һ������ˮ����Ʒ����Һ���ܹ�����SO2��CO2������ǣ�������
| A�� | �٢ڢ� | B�� | ֻ�Тڢۢ� | C�� | �ڢۢܢ� | D�� | ȫ������ |
13��������ġ� ���еġ�-OH����±ԭ��ȡ�����õĻ������Ϊ��±�����л������п��Կ�����±���ǣ�������
���еġ�-OH����±ԭ��ȡ�����õĻ������Ϊ��±�����л������п��Կ�����±���ǣ�������
 ���еġ�-OH����±ԭ��ȡ�����õĻ������Ϊ��±�����л������п��Կ�����±���ǣ�������
���еġ�-OH����±ԭ��ȡ�����õĻ������Ϊ��±�����л������п��Կ�����±���ǣ�������| A�� | CHBr=CHCH3 | B�� | CCl4 | C�� | COCl2 | D�� | CH2ClCOOH |
 ��
��



 +HCl
+HCl

 ���������֣�
���������֣�