��Ŀ����
������ͭ�Ǵ���ˮ�潢������Ϳ�����Ҫԭ�ϡ�ijС����������о�������д���пհס�
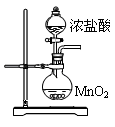
ʵ��1��������ͭ����ȡ������ͭ���������Ǻ�����������ͭ����Һ��Ӧ��ȡ�����ױ������������Ʋ���ʱ��������CuO���ɡ�
��1��ʵ������ȡ������ͭ����Һ�����ӷ���ʽΪ____________��
��2��ʵ�����ô˷�����ȡ���������������ͭ���壬��Ҫ�IJ����������Թܡ��ƾ��ơ��ձ�____________��____________��
��3����Ҫ̽���÷�Ӧ����������¶ȣ�Ӧѡ�õļ��ȷ�ʽΪ____________��
ʵ��2���ⶨ������ͭ�Ĵ���
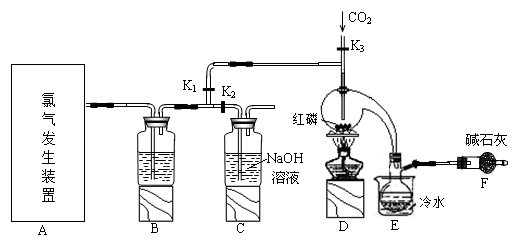
����1����ȡʵ��1���ù���m g����������װ�ý���ʵ�顣
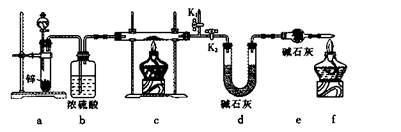
��4��װ��a�����ӵ�����____________���ѧʽ����
��5��ͨ������������������ܴﵽʵ��Ŀ�ĵ���____________��
��6���������鴿��ȼװ��c�оƾ���֮ǰ��Ҫ��K1��K2���еIJ����� ____________
����2����ʵ��l���ù���mg��������ϡ���ᣬ�����ˡ�ϴ�ӡ������Ƴ�����������������ϣ�Cu2O+2H+=Cu2++Cu+H2O��
��7���жϾ������������IJ������Ƿ�����ȫ����IJ���������__________________________________��
��8����ʵ�����ò�����Ϊng�������Ʒ��������ͭ����������Ϊ________________��
ʵ��1��������ͭ����ȡ������ͭ���������Ǻ�����������ͭ����Һ��Ӧ��ȡ�����ױ������������Ʋ���ʱ��������CuO���ɡ�
��1��ʵ������ȡ������ͭ����Һ�����ӷ���ʽΪ____________��
��2��ʵ�����ô˷�����ȡ���������������ͭ���壬��Ҫ�IJ����������Թܡ��ƾ��ơ��ձ�____________��____________��
��3����Ҫ̽���÷�Ӧ����������¶ȣ�Ӧѡ�õļ��ȷ�ʽΪ____________��
ʵ��2���ⶨ������ͭ�Ĵ���
����1����ȡʵ��1���ù���m g����������װ�ý���ʵ�顣

��4��װ��a�����ӵ�����____________���ѧʽ����
��5��ͨ������������������ܴﵽʵ��Ŀ�ĵ���____________��
| A����Ӧǰ��װ��a������ |
| B��װ��c��ַ�Ӧ�����ù�������� |
| C����Ӧǰ��װ��d������ |
| D����Ӧǰ��װ��e������ |
����2����ʵ��l���ù���mg��������ϡ���ᣬ�����ˡ�ϴ�ӡ������Ƴ�����������������ϣ�Cu2O+2H+=Cu2++Cu+H2O��
��7���жϾ������������IJ������Ƿ�����ȫ����IJ���������__________________________________��
��8����ʵ�����ò�����Ϊng�������Ʒ��������ͭ����������Ϊ________________��
��1��Cu2++2OH��=Cu(OH)2����2�֣�
��2��©��������������1�֣���2�֣�
��3��ˮԡ���ȣ�2�֣�
��4��H2SO4��1�֣�
��5��BC��ѡ��1����ȫѡ����2�֣�
��6����K2���ر�K1��2�֣�
��7�����������ٴθ���������ֱ�������������������ͬ�������������𰸣���2�֣�
��8��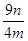 ��100% �������������𰸣���2�֣�
��100% �������������𰸣���2�֣�
��2��©��������������1�֣���2�֣�
��3��ˮԡ���ȣ�2�֣�
��4��H2SO4��1�֣�
��5��BC��ѡ��1����ȫѡ����2�֣�
��6����K2���ر�K1��2�֣�
��7�����������ٴθ���������ֱ�������������������ͬ�������������𰸣���2�֣�
��8��
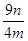 ��100% �������������𰸣���2�֣�
��100% �������������𰸣���2�֣������������1��ʵ���ҳ��ÿ�����ͭ����Һ���������Ʒ�Ӧ��ȡ������ͭ����ӦʽΪCu2++2OH��=Cu(OH)2������2��������������������ͭ���ֱ�Ӽ�����Ҫ�Թܡ��ƾ��ƣ���Һ���з����Cu2O������Ҫ�ձ���©��������������3��̽���÷�Ӧ����������¶ȣ�����ѡ��ˮԡ���ȣ���Ҫ�¶ȼƲ���ˮԡ���¶ȣ���4��п����������ᡢ�ѻӷ����ᷴӦ������ȡ��������aװ��Ӧ����ϡH2SO4����5��װ��d���ӵ�����Դ��c�з�Ӧ������ˮ���ɴ˿��Լ������Ʒ����Ԫ�ص�������c��Ӧ�����ù������������ͭ��������������������Ԫ�ص��������Բ��������ͭ�Ĵ��ȣ���BC��ȷ����6����K2���ر�K1��ͨ����һ������ټ���cװ�ã���7�����������ٴθ���������ֱ�������������������ͬ�������������𰸣���˵���������Ƿ�����ȫ�����8����m/M��֪n(Cu)=n/64mol����Cu2O+2H+=Cu2++Cu+H2O��֪n(Cu2O)=n/64mol����n?M��֪m(Cu2O)=144n/64g=9n/4g������Ʒ��Cu2O�Ĵ���Ϊ9n/4m��100%��

��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д�
�����Ŀ
 2MgO����3Mg+N2
2MgO����3Mg+N2 Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2 2MgO+C��
2MgO+C�� MgO+H2���� ��Mg3N2 +6H2O
MgO+H2���� ��Mg3N2 +6H2O 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3��