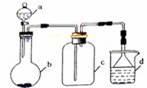
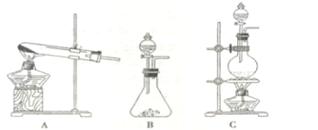
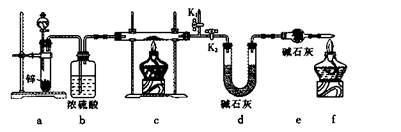
��Ŀ����
������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ(Mg3N2)����֪ʵ���п��ܻᷢ�����з�Ӧ��
��2Mg+O2 2MgO����3Mg+N2
2MgO����3Mg+N2 Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2 2MgO+C��
2MgO+C��
��Mg+H2O MgO+H2���� ��Mg3N2 +6H2O
MgO+H2���� ��Mg3N2 +6H2O 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3��
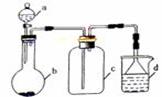
�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ(þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ������������)��

�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��D��E�⣬��Ӧѡ���װ���� ������ĸ���ţ���ѡ��װ��DĿ��Ϊ_____________________________ ��
��2��ͨ����Ӧ�ȵ�ȼ ���ľƾ��ƣ����ͬʱ��ȼA��Fװ�õľƾ��ƣ�����ʹʵ���� ���ƫ�ߡ���ƫ�͡���ԭ��
��3�������һ��ʵ�飬��֤������Mg3N2��д���������衢����ͽ��ۣ�
_____________________________________________________________________
��2Mg+O2
 2MgO����3Mg+N2
2MgO����3Mg+N2 Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2 2MgO+C��
2MgO+C����Mg+H2O
 MgO+H2���� ��Mg3N2 +6H2O
MgO+H2���� ��Mg3N2 +6H2O 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3���ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ(þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ������������)��

�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��D��E�⣬��Ӧѡ���װ���� ������ĸ���ţ���ѡ��װ��DĿ��Ϊ_____________________________ ��
��2��ͨ����Ӧ�ȵ�ȼ ���ľƾ��ƣ����ͬʱ��ȼA��Fװ�õľƾ��ƣ�����ʹʵ���� ���ƫ�ߡ���ƫ�͡���ԭ��
��3�������һ��ʵ�飬��֤������Mg3N2��д���������衢����ͽ��ۣ�
_____________________________________________________________________
��1��BF ��2�֣� D Ŀ���dz�ȥ�����е�CO2�����ⷴӦ�۷�������2�֣�
��2��F����1�֣�ƫ�ͣ���2�֣����װ��F�еĻ�ԭ����û�дﵽ��Ӧ�¶�ʱ���������ܳ�����������ͬþ��Ӧ����ʹ����þ�л�������þ����2�֣�
��3��ȡ������������Թ��У��μ�����ˮ������ʪ�ĺ�ɫʯ����ֽ�����Թܿڣ�����Թ��е���Һ���ֻ��ǣ���ɫʯ����ֽ�����������֤���е���þ���ɡ���2�֣�
��2��F����1�֣�ƫ�ͣ���2�֣����װ��F�еĻ�ԭ����û�дﵽ��Ӧ�¶�ʱ���������ܳ�����������ͬþ��Ӧ����ʹ����þ�л�������þ����2�֣�
��3��ȡ������������Թ��У��μ�����ˮ������ʪ�ĺ�ɫʯ����ֽ�����Թܿڣ�����Թ��е���Һ���ֻ��ǣ���ɫʯ����ֽ�����������֤���е���þ���ɡ���2�֣�
�����������1���÷�Ӧ��Ϊ�˵õ������ĵ���þ�������Ӱ��IJ��������ˮ����������ȥ����װ��B��������ȥˮ�����ģ�����A֮ǰ��F����������ȥ������D Ŀ���dz�ȥ�����е�CO2�����ⷴӦ�۷�����
��2����һװ���У��ܶ��˿��ܻῼ�ǵ�Fװ����������ȥˮ�����ģ������Ļ�����Ӧ������������ô�죬������װ����������ȥ�����ġ����װ��F�еĻ�ԭ����û�дﵽ��Ӧ�¶�ʱ���������ܳ�����������ͬþ��Ӧ����ʹ����þ�л�������þ����2�֣�
��3����֤������Mg3N2���������õ���þ��������Ӧ��ȡ������������Թ��У��μ�����ˮ������ʪ�ĺ�ɫʯ����ֽ�����Թܿڣ�����Թ��е���Һ���ֻ��ǣ���ɫʯ����ֽ�����������֤���е���þ���ɡ�

��ϰ��ϵ�д�
�����Ŀ