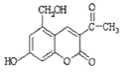
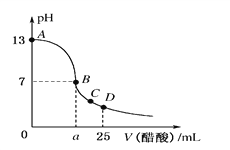
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ Β―ι≤βΒΟ0.5molΓΛL1CH3COONa»ή“ΚΓΔ0.5molΓΛL1CuSO4»ή“Κ“‘ΦΑH2OΒΡpHΥφΈ¬Ε»±δΜ·ΒΡ«ζœΏ»γΆΦΥυ ΨΓΘœ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «Θ® Θ©

A.Β±Έ¬Ε»ΈΣ50Γφ ±Θ§¥ΩΥ°÷–c(H+)=c(OH)
B.ΥφΈ¬Ε»…ΐΗΏΘ§CuSO4»ή“ΚΒΡc(H+)‘ω¥σ
C.ΥφΈ¬Ε»…ΐΗΏΘ§CH3COONa»ή“ΚΒΡpH±δΜ·”κKwΗΡ±δ”–ΙΊ
D.ΥφΈ¬Ε»…ΐΗΏΘ§CH3COONa»ή“ΚΚΆCuSO4»ή“ΚΒΡpHΨυΫΒΒΆΘ§ «“ρΈΣCH3COO-ΓΔCu2+Υ°ΫβΤΫΚβ“ΤΕ·ΖΫœρ≤ΜΆ§
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩ
Υ°ΒΡΒγάκΈΣΈϋ»»Ιΐ≥ΧΘ§…ΐΗΏΈ¬Ε»Θ§¥ΌΫχΥ°ΒΡΒγάκΘΜ―ΈάύΥ°ΫβΈΣΈϋ»»Ιΐ≥ΧΘ§…ΐΗΏΈ¬Ε»¥ΌΫχ―ΈάύΥ°ΫβΘ§Ψί¥ΥΫβΧβΓΘ
AΘ°Έό¬έ¥Π”ΎΕύ…ΌΕ»Θ§¥ΩΥ°÷–ΕΦ¥φ‘Ύc(H+)=c(OH)Θ§A’ΐ»ΖΘΜ
BΘ°ΥφΈ¬Ε»…ΐΗΏΘ§¥ΌΫχCuSO4»ή“ΚΒΡ÷–ΒΡCu2+Υ°ΫβΘ§Cu2+2H2O![]() Cu(OH)2+2H+Θ§c(H+)‘ω¥σΘ§B’ΐ»ΖΘΜ
Cu(OH)2+2H+Θ§c(H+)‘ω¥σΘ§B’ΐ»ΖΘΜ
CΘ°…ΐΗΏΈ¬Ε»Θ§¥ΌΫχΥ°ΒΡΒγάκΘ§Kw±δ¥σΘ§c(OH- )‘ω¥σΘΜ…ΐΗΏΈ¬Ε»Θ§¥ΌΫχ¥ΉΥαΗυΥ°ΫβCH3COO-+H2O![]() CH3COOH+OH-Θ§c(OH- )‘ω¥σΘ§ΝΫ’ΏΙ≤Ά§Ής”Ο ΙpHΖΔ…ζ±δΜ·Θ§ pH±δ¥σΘ§Ι CH3COONa»ή“ΚΒΡpH±δΜ·”κKwΗΡ±δ”–ΙΊΘ§C’ΐ»ΖΘΜ
CH3COOH+OH-Θ§c(OH- )‘ω¥σΘ§ΝΫ’ΏΙ≤Ά§Ής”Ο ΙpHΖΔ…ζ±δΜ·Θ§ pH±δ¥σΘ§Ι CH3COONa»ή“ΚΒΡpH±δΜ·”κKwΗΡ±δ”–ΙΊΘ§C’ΐ»ΖΘΜ
DΘ°―ΈάύΥ°ΫβΈΣΈϋ»»Ιΐ≥ΧΘ§…ΐΗΏΈ¬Ε»¥ΌΫχ―ΈάύΥ°ΫβΘ§CH3COO-ΓΔCu2+Υ°ΫβΤΫΚβ“ΤΕ·ΖΫœρœύΆ§Θ§D¥μΈσΘΜ
¥πΑΗ―ΓDΓΘ