��Ŀ����
����Aֻ����������һ�ȴ���B��C��D��C�Ľṹ��ʽ�� ��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E�����Ϸ�Ӧ��B�Ľ�һ����Ӧ����ͼ��ʾ��
��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E�����Ϸ�Ӧ��B�Ľ�һ����Ӧ����ͼ��ʾ��
��ش�
��1��A�Ľṹ��ʽ��__________________________��
��2��F�Ĺ�����������_______��E��һ��������ת��ΪB����_______��Ӧ���Ӧ���ͣ���
��3��Bת��ΪE�Ļ�ѧ����ʽΪ��______________________________________________________��
G��H��Ӧ�����ӷ���ʽΪ��____________________________________________________________��
��4��д����F������̼ԭ����ΪFͬϵ�������ͬ���칹��Ľṹ��ʽ��
_____________________________________________________________________________________��
��1��(CH3)3C��CH2CH3��2�֣�����2���ǻ����ӳɣ�����1�֣�
��3��(CH3)3C��CH2CH2Cl+NaOH (CH3)3CCH=CH2+NaCl+H2O����2�֣�
(CH3)3CCH=CH2+NaCl+H2O����2�֣�
(CH3)3CCH2CHO+2Ag(NH3)2++2OH- (CH3)3CCH2COO-+2Ag��+ NH4++3NH3+H2O����2�֣�
(CH3)3CCH2COO-+2Ag��+ NH4++3NH3+H2O����2�֣�
��4��CH3CH2CH2CH2OH��CH3CH2CH(OH)CH3��(CH3)2CHCH2OH��(CH3)2C(OH)CH3��
��4�֣�ÿ1�֣�
�����������������Aֻ����������һ��ȡ������B��C��D��C�Ľṹ��ʽ��CH3CH2C(CH3)2CH2Cl������A�Ľṹ��ʽΪ(CH3)3CCH2CH3��B��D�ֱ���ǿ��Ĵ���Һ���ȣ���ֻ�ܵõ��л�������E����E�Ľṹ��ʽΪ(CH3)3CCH��CH2��B����������ˮ��Һ������F��FΪ����F��������G��G����������Һ��Ӧ��G����ȩ����CHO����B��F��G��H��D�Ľṹ��ʽ�ֱ�Ϊ(CH3)3CCH2CH2Cl��(CH3)3CCH2CH2OH��(CH3)3CCH2CHO��(CH3)3CCH2COOH��(CH3)3CCH2ClCH3����
��1���������Ϸ�����֪��A�Ľṹ��ʽ��(CH3)3C��CH2CH3��
��2������F�Ľṹ��ʽ��֪�������к��еĹ������������ǻ���E�����к���̼̼˫�����ܺ��Ȼ��ⷢ���ӳɷ�Ӧ����B����E��һ��������ת��ΪB�ķ�Ӧ�Ǽӳɷ�Ӧ��
��3��Bת��ΪE��±��������ȥ��Ӧ�����Bת��ΪE�Ļ�ѧ����ʽΪ(CH3)3C��CH2CH2Cl��NaOH (CH3)3CCH=CH2��NaCl��H2O��G��H��Ӧ��������Ӧ���䷴Ӧ�����ӷ���ʽΪ(CH3)3CCH2CHO+2Ag(NH3)2++2OH-
(CH3)3CCH=CH2��NaCl��H2O��G��H��Ӧ��������Ӧ���䷴Ӧ�����ӷ���ʽΪ(CH3)3CCH2CHO+2Ag(NH3)2++2OH- (CH3)3CCH2COO-+2Ag��+ NH4++3NH3+H2O��
(CH3)3CCH2COO-+2Ag��+ NH4++3NH3+H2O��
��4����F������̼ԭ����ΪFͬϵ���˵�����л����Ǻ���4��̼ԭ�ӵı���һԪ��������ܵĽṹ��ʽΪCH3CH2CH2CH2OH��CH3CH2CH(OH)CH3��(CH3)2CHCH2OH��(CH3)2C(OH)CH3��
���㣺�����л����ƶϡ������š��л���Ӧ���͡�ͬ���칹���ж��Լ�����ʽ����д��

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�ij�о�С���Լױ�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�ҽҩ�м���F��Y��
��֪��
��ش��������⣺
��1�������й�F��˵����ȷ���� ��
| A������ʽ��C7H7NO2Br | B�����γ����� |
| C���ܷ���ȡ����Ӧ�����۷�Ӧ | D��1 mol�� F�����Ժ�2 mol NaOH��Ӧ |
��3�� B��C�Ļ�ѧ����ʽ�� ���ںϳ�F�Ĺ����У�B��C���費��ʡ�ԣ������� ��
��4��D��E��Ӧ������Լ��� ��
��5��д��ͬʱ��������������A��ͬ���칹��Ľṹ��ʽ ��д��3������
�ٱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�� �ڷ����к���
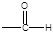
��6����X����ϩΪԭ�Ͽɺϳ�Y������ƺϳ�·�ߣ����Լ����ܼ���ѡ����
ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��
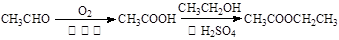
��11�֣�����21���ͣ��ҹ��ļ�����Ⱦ�¼��У�����ʯ��˫����������ը��Ψһ��ɹ���Ӱ����¼�����100�ֺ��б����ױ����������ͱ�����  ������Ⱦ��ķ�ˮ�����ɻ��������վ�����˹�뺣��
������Ⱦ��ķ�ˮ�����ɻ��������վ�����˹�뺣��
��1��д������������Ⱦ���к��еĹ����ŵĽṹ��ʽ ��
��2�������ױ����������ͱ���4�����ʵĹ�ϵΪ ��
| A��ͬϵ�� | B��ͬ���칹�� | C�������� | D�������廯���� |
��
��4����д���ɱ����������Ļ�ѧ����ʽ ��
�÷�Ӧ�ķ�Ӧ������ ��
����֪�������ڹ�����Fe�ۺ�����������¿��Ƶñ�����д���÷�Ӧ�Ļ�ѧ����ʽ ��

 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɡ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɡ�
 ��ش��������⣺
��ش��������⣺





 ��
�� ��
��
